Abstract
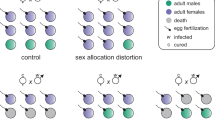
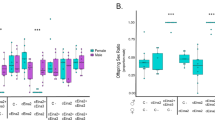
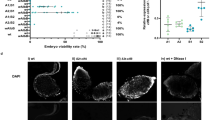
Cytoplasmic incompatibility (CI) is a phenomenon where embryonic development is disrupted–often leading to complete failure–when the female parent lacks the symbiont strain carried by the male parent. This mechanism, employed by maternally transmitted symbionts such as Wolbachia, facilitates their rapid spread within a host population. CI has significant potential as a tool for achieving population replacement or suppression, particularly targeting disease vectors and agricultural pests. While complete expression of CI is ideal for such applications, its intensity can vary. Despite extensive research on the symbiont genotypes, the influence of host genetic background on CI expression remains poorly understood. Here, we found that in the bean beetle Callosobruchus analis, the Wolbachia strain wCana2 induces weak CI in its native nuclear background but strong CI in a previously unassociated nuclear background. Crossing experiments reveal that the nuclear backgrounds of both male and female parents can significantly affect CI expression independent of Wolbachia titres in C. analis. These findings uncover novel perspectives on the host-symbiont interactions underlying CI and highlight the complexities to be addressed for its practical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Adams KL, Abernathy DG, Willett BC, Selland EK, Itoe MA, Catteruccia F (2021) Wolbachia cifB induces cytoplasmic incompatibility in the malaria mosquito vector. Nat Microbiol. 6:1575–1582. https://doi.org/10.1038/s41564-021-00998-6
Beckmann JF, Ronau JA, Hochstrasser M (2017) A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol. 2:1–7. https://doi.org/10.1038/nmicrobiol.2017.7
Beckmann JF, Sharma GD, Mendez L, Chen H, Hochstrasser M (2019) The Wolbachia cytoplasmic incompatibility enzyme CidB targets nuclear import and protamine-histone exchange factors. eLife. 8:e50026. https://doi.org/10.7554/eLife.50026
Beckmann JF, Van Vaerenberghe K, Akwa DE, Cooper BS (2021) A single mutation weakens symbiont-induced reproductive manipulation through reductions in deubiquitylation efficiency. PNAS. 118:e2113271118. https://doi.org/10.1073/pnas.211327111
Bordenstein SR, Werren JH (1998) Effects of A and B Wolbachia and host genotype on interspecies cytoplasmic incompatibility in Nasonia. Genetics. 148:1833–1844. https://doi.org/10.1093/genetics/148.4.1833
Bourtzis K (2008) Wolbachia-based technologies for insect pest population control. In: Aksoy S (eds) Transgenesis and the management of vector-borne disease. Adv Exp Med Biol. 627. Springer, New York, NY. https://doi.org/10.1007/978-0-387-78225-6_9
Boyle L, O’Neill SL, Robertson HM, Karr TL (1993) Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science. 260:1796–1799. https://doi.org/10.1126/science.8511587
Carrington LB, Lipkowitz JR, Hoffmann AA, Turelli M (2011) A re-examination of Wolbachia-induced cytoplasmic incompatibility in California Drosophila simulans. PLoS One. 6:e22565. https://doi.org/10.1371/journal.pone.0022565
Castle WE (1921) An improved method of estimating the number of genetic factors concerned in cases of blending inheritance. Science. 54:223–223. https://doi.org/10.1126/science.54.1393.223
Clancy DJ, Hoffmann AA (1997) Behavior of Wolbachia endosymbionts from Drosophila simulans in Drosophila serrata, a novel host. Am Nat. 149:975–988. https://doi.org/10.1086/286033
Cooper BS, Ginsberg PS, Turelli M, Matute DR (2017) Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics. 205:333–351. https://doi.org/10.1534/genetics.116.196238
Duron O, Bernard J, Atyame CM, Dumas E, Weill M (2012) Rapid evolution of Wolbachia incompatibility types. Proc R Soc B. 279:4473–4480. https://doi.org/10.1098/rspb.2012.1368
Edwards B, Ghedin E, Voronin D (2023) Wolbachia interferes with Zika virus replication by hijacking cholesterol metabolism in mosquito cells. Microbiol Spectr. 11:e02180-23. https://doi.org/10.1128/spectrum.02180-23
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci. 7:457–472. https://doi.org/10.1214/ss/1177011136
Gelman A, Hill J (2006) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, pp 473–477
Georghiou GP, Mellon RB (1983) Pesticide resistance in time and space. In: Georghiou GP, Saito T (eds) Pest resistance to pesticides. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-4466-7_1
Gomes FM, Hixson BL, Tyner MDW, Ramirez JL, Canepa GE, Alves e Silva TL et al. (2017) Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. PNAS. 114:12566–12571. https://doi.org/10.1073/pnas.1716181114
Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 476:454–457. https://doi.org/10.1038/nature10356
Hoffmann AA, Cooper BS (2024) Describing endosymbiont–host interactions within the parasitism–mutualism continuum. Ecol Evol. 14:e11705. https://doi.org/10.1002/ece3.11705
Kageyama D, Narita S, Imamura T, Miyanoshita A (2010) Detection and identification of Wolbachia endosymbionts from laboratory stocks of stored-product insect pests and their parasitoids. J Stored Prod Res. 46:13–19. https://doi.org/10.1016/j.jspr.2009.07.003
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc. 90:319–323. https://doi.org/10.1080/01621459.1995.10476572
Koehncke A, Telschow A, Werren JH, Hammerstein P (2009) Life and death of an influential passenger: Wolbachia and the evolution of CI-modifiers by their hosts. PLoS One. 4:e4425. https://doi.org/10.1371/journal.pone.0004425
Kondo NI, Tuda M, Toquenaga Y, Lan YC, Buranapanichpan S, Horng SB et al. (2011) Wolbachia infections in world populations of bean beetles (Coleoptera: Chrysomelidae: Bruchinae) infesting cultivated and wild legumes. Zool Sci. 28:501–508. https://doi.org/10.2108/zsj.28.501
Kyritsis GA, Augustinos AA, Livadaras I, Cáceres C, Bourtzis K, Papadopoulos NT (2019) Medfly-Wolbachia symbiosis: genotype x genotype interactions determine host’s life history traits under mass rearing conditions. BMC Biotechnol. 19:96. https://doi.org/10.1186/s12896-019-0586-7
LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD et al. (2017) Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 543:243–247. https://doi.org/10.1038/nature21391
Liang X, Tan CH, Sun Q, Zhang M, Wong PSJ, Li MI et al. (2022) Wolbachia wAlbB remains stable in Aedes aegypti over 15 years but exhibits genetic background-dependent variation in virus blocking. PNAS Nexus. 1:pgac203. https://doi.org/10.1093/pnasnexus/pgac203
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. https://doi.org/10.1006/meth.2001.1262
Martinez J, Klasson L, Welch JJ, Jiggins FM (2021) Life and death of selfish genes: comparative genomics reveals the dynamic evolution of cytoplasmic incompatibility. Mol Biol Evol. 38:2–15. https://doi.org/10.1093/molbev/msaa209
McNamara CJ, Ant TH, Harvey-Samuel T, White-Cooper H, Martinez J, Alphey L et al. (2024) Transgenic expression of cif genes from Wolbachia strain wAlbB recapitulates cytoplasmic incompatibility in Aedes aegypti. Nat Commun. 15:869. https://doi.org/10.1038/s41467-024-45238-7
Morey RD, Rouder JN, Jamil T, Urbanek S, Forner K, Ly A (2025) BayesFactor: Computation of bayes factors for common designs. R package version 0.9.12-4.7. https://CRAN.R-project.org/package=BayesFactor
Namias A, Ngaku A, Makoundou P, Unal S, Sicard M, Weill M (2024) Intra-lineage microevolution of Wolbachia leads to the emergence of new cytoplasmic incompatibility patterns. PLoS Biol. 22:e3002493. https://doi.org/10.1371/journal.pbio.3002493
Numajiri Y, Kondo NI, Toquenaga Y (2017) Melanic mutation causes a fitness decline in bean beetles infected by Wolbachia. Entomol Exp Appl 164:54–65. https://doi.org/10.1111/eea.12588
Numajiri Y, Kondo NI, Toquenaga Y, Kageyama D (2024) Intraspecies variation in cytoplasmic incompatibility intensity in the bean beetle Callosobruchus analis. Evol Ecol 38:861–870. https://doi.org/10.1007/s10682-024-10311-6
Poinsot D, Bourtzis K, Markakis G, Savakis C, Merçot H (1998) Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227–237. https://doi.org/10.1093/genetics/150.1.227
Poinsot D, Merçot H (2001) Wolbachia injection from usual to naive host in Drosophila simulans (Diptera: Drosophilidae). Eur J Entomol 98:25–30. https://doi.org/10.14411/EJE.2001.004
Ross PA, Gu X, Robinson KL, Yang Q, Cottingham E, Zhang Y et al. (2021) A wAlbB Wolbachia transinfection displays stable phenotypic effects across divergent Aedes aegypti mosquito backgrounds. Appl Environ Microbiol 87:e01264-21. https://doi.org/10.1128/AEM.01264-21
R Core Team (2024) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Riegler M, Charlat S, Stauffer C, Merçot H (2004) Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: investigating the outcomes of host-symbiont coevolution. Appl Environ Microbiol 70:273–279. https://doi.org/10.1128/AEM.70.1.273-279.2004
Schönbrodt FD, Wagenmakers EJ (2018) Bayes factor design analysis: Planning for compelling evidence. Psychon Bull Rev 25:128–142. https://doi.org/10.3758/s13423-017-1230-y
Serebrovsky AS (1928) An analysis of the inheritance of quantitative transgressive characters. Z Ver-erbungslehre 48:229–243. https://doi.org/10.1007/BF01740960
Shropshire JD, On J, Layton EM, Zhou H, Bordenstein SR (2018) One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster. PNAS 115:4987–4991. https://doi.org/10.1073/pnas.1800650115
Shropshire JD, Leigh B, Bordenstein SR (2020) Symbiont-mediated cytoplasmic incompatibility: what have we learned in 50 years? eLife 9:e61989. https://doi.org/10.7554/eLife.61989
Shropshire JD, Hamant E, Cooper BS (2021) Male age and Wolbachia dynamics: investigating how fast and why bacterial densities and cytoplasmic incompatibility strengths vary. mBio 12:e02998-21. https://doi.org/10.1128/mbio.02998-21
Shropshire JD, Hamant E, Conner WR, Cooper BS (2022) cifB-transcript levels largely explain cytoplasmic incompatibility variation across divergent Wolbachia. PNAS Nexus 1:pgac099. https://doi.org/10.1093/pnasnexus/pgac099
Sun JX, Guo Y, Zhang X, Zhu WC, Chen YT, Hong XY (2016) Effects of host interaction with Wolbachia on cytoplasmic incompatibility in the two-spotted spider mite Tetranychus urticae. Biol J Lin Soc 119:145–157. https://doi.org/10.1111/bij.12804
Sun G, Zhang M, Chen H, Hochstrasser M (2022) The CinB nuclease from wNo Wolbachia is sufficient for induction of cytoplasmic incompatibility in Drosophila. mBio 13:e03177-21. https://doi.org/10.1128/mbio.03177-21
Tang FHM, Lenzen M, McBratney A, Maggi F (2021) Risk of pesticide pollution at the global scale. Nat Geosci 14:206–210. https://doi.org/10.1038/s41561-021-00712-5
Turelli M (1994) Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500–1513. https://doi.org/10.1111/j.1558-5646.1994.tb02192.x
Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. https://doi.org/10.1038/nature10355
Wang W, Cui W, Yang H (2022) Toward an accurate mechanistic understanding of Wolbachia-induced cytoplasmic incompatibility. Environ Microbiol 24:4519–4532. https://doi.org/10.1111/1462-2920.16125
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. https://doi.org/10.1038/nrmicro1969
Wybouw N, Mortier F, Bonte D (2022) Interacting host modifier systems control Wolbachia-induced cytoplasmic incompatibility in a haplodiploid mite. Evol Lett 6:255–265. https://doi.org/10.1002/evl3.282
Yadav JS (1971) Karyological studies on the three species of Bruchidae (Coleoptera). Caryologia 24:157–166. https://doi.org/10.1080/00087114.1971.10796423
Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K (2004) Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. PNAS 101:15042–15045. https://doi.org/10.1073/pnas.0403853101
Zeng ZB (1992) Correcting the bias of Wright’s estimates of the number of genes affecting a quantitative character: a further improved method. Genetics 131:987–1001. https://doi.org/10.1093/genetics/131.4.987
Acknowledgements
This work was funded in part by Grant-in-Aids for Scientific Research (no. 23570017 to Y.T.) from the JSPS, and Cabinet Office, Government of Japan, Moonshot Research and Development Programme for Agriculture, Forestry, and Fisheries (funding agency: Bio-oriented Technology Research Advancement Institution) (no. JPJ009237 to D.K.).
Author information
Authors and Affiliations
Contributions
YN and NIK performed research. YN analyzed and interpreted data. YN, NIK, YT and DK edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research ethics statement
This study involved only unregulated invertebrate species and did not require ethical approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Sara Goodacre.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Numajiri, Y., Kondo, N.I., Toquenaga, Y. et al. Both maternal and paternal genotypes modulate Wolbachia-induced cytoplasmic incompatibility in graham bean beetles. Heredity 134, 512–518 (2025). https://doi.org/10.1038/s41437-025-00787-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41437-025-00787-5