Abstract
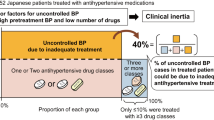
Hypertension treatment and blood pressure (BP) control reduce cardiovascular disease burden. However, prevalence of controlled BP is overall insufficient and lack of adherence to treatment is a suggested major contributor. This prospective, randomized clinical trial was designed to evaluate whether a specific 3-month (m) action plan to improve therapeutic adherence results in a decrease in BP. Patients with ambulatory 24 h-BP ≥ 130/80 mmHg despite receiving ≥2 antihypertensive drugs and with therapeutic non-compliance confirmed by antihypertensive drugs analyzed in urine were randomized (1:1) to receive a specific 3 m program to improve adherence (INT = intervention) or routine follow-up (C = control). Antihypertensive treatment was not modified and knowledge of non-adherence was only notified to patients randomized to the intervention group. Before randomization and at 3 m all patients underwent urinary screening for antihypertensive drugs and 24 h-ambulatory-BP monitoring. Forty-five patients (36% women, mean age: 58 ± 13 yr) were randomized. At 3 m, mean (95% CI) BP differences (INT vs. C) were 12.2 mmHg (4.3–20.8), adjusted-p = 0.032 and 8.7 mmHg (2.5–14.8), adjusted-p = 0.018 for 24 h-systolic and 24 h-diastolic BP, respectively. Differences (INT vs. C) for office SBP and DBP were 18.4 mmHg (6.8–30.1), adjusted-p = 0.005 and 15.7 mmHg (7.2–24.2), adjusted-p < 0.001. Non-detected antihypertensive drugs were median [IQR]: 40% [25–100] and 0% [0–20] at baseline and 3 m, respectively, in the INT group, and 33.3% [25–63.7] and 33.3% [23.8–57.9], in the C group (p < 0.001 for the 3-month between-group comparison). A combined action plan of notifying knowledge of non-adherence plus a 3-month specific nursing intervention to improve therapeutic adherence results in BP reduction in patients with inadequate therapeutic compliance.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, European Society of Cardiology,et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020;41:12–85.
Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–92.
Soriano JB, Rojas-Rueda D, Alonso J, Antó JM, Cardona PJ, Fernández E, Colaboradores de GBD en España,et al. The burden of disease in Spain: results from the Global Burden of Disease 2016. Med Clin. 2018;151:171–90.
Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–200.
Banegas JR, Gijón-Conde T. Hypertension: the most common chronic health problem in Spain. A call to action. Hipertens Riesgo Vasc. 2022;39:121–7.
Durand H, Hayes P, Morrissey EC, Newell J, Casey M, Murphy AW, et al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35:2346–57.
Mennini FS, Marcellusi A, von der Schulenburg JM, Gray A, Levy P, Sciattella P, et al. Cost of poor adherence to anti-hypertensive therapy in five European countries. Eur J Health Econ. 2015;16:65–72.
Lee EKP, Poon P, Yip BHK, Bo Y, Zhu MT, Yu CP, et al. Global burden, regional differences, trends, and health consequences of medication nonadherence for hypertension during 2010 to 2020: a meta-analysis involving 27 million patients. J Am Heart Assoc. 2022;11:e026582.
Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine. 2017;96:e5641.
Marshall IJ, Wolfe CD, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ. 2012;345:e3953.
Hamdidouche I, Jullien V, Boutouyrie P, Billaud E, Azizi M, Laurent S. Routine urinary detection of antihypertensive drugs for systematic evaluation of adherence to treatment in hypertensive patients. J Hypertens. 2017;35:1891–8.
Choudhry NK, Kronish IM, Vongpatanasin W, Ferdinand KC, Pavlik VN, Egan BM, American Heart Association Council on Hypertension; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology, et al. Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension. 2022;79:e1–e14.
Sutherland JJ, Morrison RD, McNaughton CD, Daly TM, Milne SB, Daniels JS, et al. Assessment of patient medication adherence, medical record accuracy, and medication blood concentrations for prescription and over-the-counter medications. JAMA Netw Open. 2018;1:e184196.
Hill MN, Miller NH, Degeest S, Materson BJ, Black HR, Izzo JL,Jr, American Society of Hypertension Writing Group, et al. Adherence and persistence with taking medication to control high blood pressure. J Am Soc Hypertens. 2011;5:56–63.
Allemann SS, Nieuwlaat R, Navarro T, Haynes B, Hersberger KE, Arnet I. Congruence between patient characteristics and interventions may partly explain medication adherence intervention effectiveness: an analysis of 190 randomized controlled trials from a Cochrane systematic review. J Clin Epidemiol. 2017;91:70–9.
Peeters LEJ, Kappers MHW, Hesselink DA, van der Net JB, Hartong SCC, van de Laar R, et al. Antihypertensive drug concentration measurement combined with personalized feedback in resistant hypertension: a randomized controlled trial. J Hypertens. 2024;42:169–78.
Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010;15:225–8.
Lawson AJ, Shipman KE, George S, Dasgupta I. A novel ‘Dilute-and-Shoot’ liquid chromatography-tandem mass spectrometry method for the screening of antihypertensive drugs in urine. J Anal Toxicol. 2016;40:17–27.
Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124:1124–40.
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–7.
Izeogu C, Kalinowski J, Schoenthaler A. Strategies to improve adherence to anti-hypertensive medications: a narrative review. Curr Hypertens Rep. 2020;22:105.
Chapman RH, Ferrufino CP, Kowal SL, Classi P, Roberts CS. The cost and effectiveness of adherence-improving interventions for antihypertensive and lipid-lowering drugs*. Int J Clin Pract. 2010;64:169–81.
van der Laan DM, Langendoen-Gort M, Nijpels G, Boons CCLM, Elders PJM, Hugtenburg JG. Implementation fidelity of an intervention programme to enhance adherence to antihypertensive medication in Dutch community pharmacies. Int J Clin Pharm. 2019;41:1031–46.
Chan AHY, Foot H, Pearce CJ, Horne R, Foster JM, Harrison J. Effect of electronic adherence monitoring on adherence and outcomes in chronic conditions: a systematic review and meta-analysis. PLoS ONE. 2022;17:e0265715.
Hedegaard U, Kjeldsen LJ, Pottegård A, Henriksen JE, Lambrechtsen J, Hangaard J, et al. Improving medication adherence in patients with hypertension: a randomized trial. Am J Med. 2015;128:1351–61.
Choudhry NK, Isaac T, Lauffenburger JC, Gopalakrishnan C, Lee M, Vachon A, et al. Effect of a remotely delivered tailored multicomponent approach to enhance medication taking for patients with hyperlipidemia, hypertension, and diabetes: the STIC2IT Cluster Randomized Clinical Trial. JAMA Intern Med. 2018;178:1182–9.
Mehta SJ, Volpp KG, Troxel AB, Day SC, Lim R, Marcus N, et al. Electronic pill bottles or bidirectional text messaging to improve hypertension medication adherence (Way 2 Text): a randomized clinical trial. J Gen Intern Med. 2019;34:2397–404.
Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu LM, Brennan T, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation. 2016;133:592–600.
Morgado M, Rolo S, Castelo-Branco M. Pharmacist intervention program to enhance hypertension control: a randomised controlled trial. Int J Clin Pharm. 2011;33:132–40.
Polychronopoulou E, Burnier M, Ehret G, Schoenenberger-Berzins R, Berney M, Ponte B, et al. Assessment of a strategy combining ambulatory blood pressure, adherence monitoring and a standardised triple therapy in resistant hypertension. Blood Press. 2021;30:332–40.
Staplin N, de la Sierra A, Ruilope LM, Emberson JR, Vinyoles E, Gorostidi M, et al. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59 124 patients. Lancet. 2023;401:2041–50.
Acknowledgements
We would like to thank Laia Fontdevila, assistant nursing technician at the Specialized Center for Hypertension, Hospital del Mar, for her support and dedication, as well as Juan José Hernández and Laura Andolza from the Laboratori de Referència de Catalunya for their crucial role in the implementation and development of laboratory methodology to detect antihypertensive drugs in urine. We also thank Dr Josep Bellmunt and Dr Ernest Vinyoles for their contribution to this study.
Funding
This project (PI16/01356) was funded in part by a research grant to AO by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveras, A., Vázquez, S., Vega, M.V. et al. Improvement of non-adherence and reduction of BP values in patients with difficult-to-treat hypertension: the ATHAN clinical trial. Hypertens Res 47, 2864–2873 (2024). https://doi.org/10.1038/s41440-024-01748-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01748-x
Keywords
This article is cited by
-
How to deal with a hypertensive patient who has documented non-adherence to the prescribed antihypertensive therapy?
Hypertension Research (2024)