Abstract
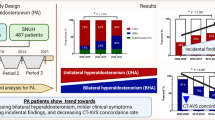
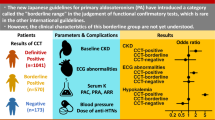
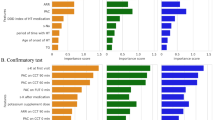
This study aims to evaluate the prevalence of unilateral hyperaldosteronism (UHA) and its clinical characteristics in patients with primary aldosteronism (PA), diagnosed using plasma aldosterone concentration (PAC) measured by chemiluminescent enzyme immunoassay (CLEIA). We retrospectively analyzed data of 199 PA patients from the Japan Primary Aldosteronism Study II (JPAS II) dataset, including patients who underwent adrenal venous sampling (AVS) and the captopril challenge test (CCT) and/or saline infusion test (SIT), with PAC measured by CLEIA. We focused on two categories: confirmed PA, where patients exhibit clear biochemical evidence of the disorder, and borderline PA, where patients present with marginal biochemical indicators, as outlined in the Japan Endocrine Society’s clinical practice guideline for the diagnosis and management of PA. In confirmed PA cases, over the half of patients was UHA, while approximately 15 to 20% of borderline cases were found to be UHA. The prevalence of hypokalemia was identified as predictor of UHA among borderline cases. Among borderline cases with no hypokalemia and adrenal nodules on CT imaging, only 6 to 8% of patients were found to have UHA. Notably, some patients exhibited UHA despite negative results on one test but confirmed result on the other, particularly those with hypokalemia or adrenal nodules on CT imaging. In conclusion, the findings validate the importance of AVS in confirmed PA cases and the need for careful assessment in borderline cases. When feasible, conducting both CCT and SIT, and interpreting their results alongside other clinical indicators, could provide a more comprehensive assessment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, et al. Japan endocrine society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J. 2022;69:327–59.
Morimoto R, Ono Y, Tezuka Y, Kudo M, Yamamoto S, Arai T, et al. Rapid screening of primary aldosteronism by a novel chemiluminescent immunoassay. Hypertension. 2017;70:334–41.
Teruyama K, Naruse M, Tsuiki M, Kobayashi H. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. J Hum Hypertens. 2022;36:77–85.
National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan. https://unit.aist.go.jp/nmij/. Accessed 23 December 2023.
Nishikawa T, Omura M, Kawaguchi M, Takatsu A, Satoh F, Ito S, et al. Calibration and evaluation of routine methods by serum certified reference material for aldosterone measurement in blood. Endocr J. 2016;63:1065–80.
Kobayashi H, Nakamura Y, Abe M, Tanabe A, Sone M, Katabami T, et al. Impact of a change to a novel chemiluminescent immunoassay for measuring plasma aldosterone on the diagnosis of primary aldosteronism. Endocr J. 2023;70:489–500.
Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103:3965–73.
Chang YL, Chen GY, Lee BC, Chen PT, Liu KL, Chang CC, et al. Optimizing adrenal vein sampling in primary aldosteronism subtyping through LC-MS/MS and secretion ratios of aldosterone, 18-oxocortisol, and 18-hydroxycortisol. Hypertens Res. 2023;46:1983–94.
Katabami T, Fukuda H, Tsukiyama H, Tanaka Y, Takeda Y, Kurihara I, et al. Clinical and biochemical outcomes after adrenalectomy and medical treatment in patients with unilateral primary aldosteronism. J Hypertens. 2019;37:1513–20.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–74.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–66.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Sartori M, Calò LA, Mascagna V, Realdi A, Macchini L, Ciccariello L, et al. Aldosterone and refractory hypertension: a prospective cohort study. Am J Hypertens. 2006;19:373–9.
Shibata H, Itoh H. Mineralocorticoid receptor- associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–23.
Tanaka A, Shibata H, Node K. Suspected borderline aldosteronism in hypertension: the next target? J Am Coll Cardiol. 2020;76:759–60.
Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20.
Parksook WW, Brown JM, Omata K, Tezuka Y, Ono Y, Satoh F et al. The spectrum of dysregulated aldosterone production: an international human physiology study. J Clin Endocrinol Metab. 2024:dgae145. Online ahead of print.
Turcu AF, Tezuka Y, Lim JS, Salman Z, Sehgal K, Liu H, et al. Multifocal, asymmetric bilateral primary aldosteronism cannot be excluded by strong adrenal vein sampling lateralization: an international retrospective cohort study. Hypertension. 2024;81:604–13.
Tetti M, Brüdgam D, Burrello J, Udager AM, Riester A, Knösel T et al. Unilateral primary aldosteronism: long-term disease recurrence after adrenalectomy. Hypertension. 2024;81:936–45.
Acknowledgements
We thank Daisuke Taura (Kyoto University), Kenji Oki (Hiroshima University), Yutaka Takahashi (Nara Medical University), Mika Tsuiki (National Hospital Organization Kyoto Medical Center), Minemori Watanabe (Okazaki City Hospital), Koichi Tamura (Yokohama City University) for collecting the clinical data, and Yuko Yasuda from the secretariat for her invaluable support and contributions.
JPAS II Study Group
Kenichi Yokota14, Masakatsu Sone14, Takuyuki Katabami6, Keiichiro Nakamae13, Mitsuhide Naruse13, Toshifumi Nakamura2, Akiyo Tanabe15, Daisuke Taura16, Yoshihiro Ogawa17, Koichi Yamamoto3, Takashi Yoneda9, Masanori Murakami11, Tetsuya Yamada11, Katsutoshi Takahashi8, Hiroki Kobayashi1, Takamasa Ichijo18, Norio Wada7, Kohei Kamemura19, Yuichi Fujii20, Yuichiro Yoshikawa21, Yasushi Miyazaki21, Shintaro Okamura22, Shigeatsu Hashimoto23, Minemori Watanabe24, Shoichiro Izawa4, Mika Tsuiki25, Hiromasa Goto26, Miki Kakutani27, Kouichi Tamura28, Nobuhito Hirawa29, Takehiro Kato30, Yutaka Takahashi31, Ryuji Okamoto10, Kazutoshi Miyashita32, Kihei Yoneyama33, Michio Otsuki34
Funding
JPAS and JRAS were supported by the research grant from the Japan Agency for Medical Research and Development (AMED) under Grant Number JP17ek0109122 and JP20ek0109352. This study was partly supported by a Grant-in-Aid from the Ministry of Health, Labour, and Welfare, Japan (No. 23FC0201 for research on intractable adrenal disorders).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, H., Nakamura, Y., Abe, M. et al. Prevalence of unilateral hyperaldosteronism in primary aldosteronism: impact of a novel chemiluminescent immunoassay for measuring plasma aldosterone in Japan. Hypertens Res 47, 3035–3044 (2024). https://doi.org/10.1038/s41440-024-01786-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01786-5