Abstract
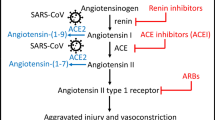
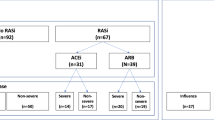
We examined the relationship between RAAS inhibitor use and Omicron infection in mildly symptomatic patients. This retrospective case-control observational study included 50,121 patients with mild Omicron infection from the largest “Fangcang” shelter hospital in Shanghai between April 9, 2022, and May 21, 2022. Using 1:1 propensity score matching (PSM), we classified 4394 COVID-19 patients into hypertension and non-hypertension groups, and 406 hypertensive patients into RAAS inhibitor and non-RAAS inhibitor groups. The risk of initial symptoms of infection, cumulative negative conversion rates, time to nucleic-acid negative conversion, and viral loads were compared. In the hypertension group, the median number of days for nucleic-acid negative conversion was 7.0 (IQR, 5.0–9.0), which was greater than non-hypertensive group (median (IQR) 6.0 (4.0–8.0), P < 0.001, Cohen’s d = 0.29); the mean and minimum cycle threshold values (CT-values) were significantly lower (P < 0.001, Cohen’s d = 0.23). In the RAAS inhibitors group, the median number of days for nucleic-acid negative conversion was 7.0 days (IQR, 5.0–9.0), which was shorter than the non-RAAS inhibitors group ([median (IQR)] 8.0 (6.0–10.0), P < 0.001, Cohen’s d = 0.34), the cumulative negative conversion rates at 3–8 days being all higher than non-RAAS inhibitors groups (P < 0.05). The most significant difference in negative conversion rate between the RAAS inhibitor and non-RAAS inhibitor group was on the 4th day. No significant difference was observed in mean and minimum CT-values from RAAS inhibitor and non-RAAS inhibitor groups (P > 0.05). RAAS inhibitor use in patients with hypertension is associated with nucleic-acid negative conversion duration and negative conversion rate. RAAS inhibitors clearly do not aggravate or prolong COVID-19.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
03 October 2025
The original online version of this article was revised: Author name spelling has been corrected. In the article “Effect of the renin–angiotensin–aldosterone system inhibitors on time to nucleic acid negative conversion in hypertensive patients with SARS-CoV-2 omicron infection: a propensity score matching study” by Ru Wen et al., published in Hypertension Research (2025, 48:273–283), the name of the second author was incorrectly spelled as “Jinwen Li.” The correct spelling is “Jingwen Li.” The authors apologize for this error. The original article has been corrected.
03 October 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41440-025-02386-7
References
Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–25. https://doi.org/10.1016/j.biopha.2017.07.091.
Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182:50–8.e58. https://doi.org/10.1016/j.cell.2020.05.027.
Minato T, Nirasawa S, Sato T, Yamaguchi T, Hoshizaki M, Inagaki T, et al. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat Commun. 2020;11:1058. https://doi.org/10.1038/s41467-020-14867-z.
Bao X, Zhu W, Yuan W, Zhu X, Yan Y, Tang H, et al. Design, synthesis and evaluation of novel potent angiotensin II receptor 1 antagonists. Eur J Med Chem. 2016;123:115–27. https://doi.org/10.1016/j.ejmech.2016.07.023.
Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis 2021;40:905–19. https://doi.org/10.1007/s10096-020-04138-6.
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–74. https://doi.org/10.1161/circresaha.120.317015.
Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228–48. https://doi.org/10.1002/path.5471.
Young MJ, Clyne CD, Chapman KE. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol. 2020;247:R45–62. https://doi.org/10.1530/joe-20-0260.
Sen R, Sengupta D, Mukherjee A. Mechanical dependency of the SARS-CoV-2 virus and the renin-angiotensin-aldosterone (RAAS) axis: a possible new threat. Environ Sci Pollut Res Int. 2022;29:62235–47. https://doi.org/10.1007/s11356-021-16356-2.
Zhang J, Wang M, Ding W, Wan J. The interaction of RAAS inhibitors with COVID-19: current progress, perspective and future. Life Sci. 2020;257:118142. https://doi.org/10.1016/j.lfs.2020.118142.
Angeli F, Zappa M, Verdecchia P. Rethinking the role of the renin-angiotensin system in the pandemic era of SARS-CoV-2. J Cardiovasc Dev Dis. 2023;10:14. https://doi.org/10.3390/jcdd10010014.
Liu D, Li YZ, Wu H. Could renin-angiotensin-aldosterone system inhibitors be used for hypertensive patients with coronavirus disease 2019? J Hypertens. 2020;38:1191–2. https://doi.org/10.1097/HJH.0000000000002474.
Gault N, Esposito-Farèse M, Revest M, Inamo J, Cabié A, Polard É, et al. Chronic use of renin-angiotensin-aldosterone system blockers and mortality in COVID-19: a multicenter prospective cohort and literature review. Fundam Clin Pharmacol. 2021;35:1141–58. https://doi.org/10.1111/fcp.12683.
Pantazi I, Al-Qahtani AA, Alhamlan FS, Alothaid H, Matou-Nasri S, Sourvinos G, et al. SARS-CoV-2/ACE2 interaction suppresses IRAK-M expression and promotes pro-inflammatory cytokine production in macrophages. Front Immunol. 2021;12:683800. https://doi.org/10.3389/fimmu.2021.683800.
Kuriakose S, Uzonna JE. Diminazene aceturate (Berenil), a new use for an old compound? Int Immunopharmacol. 2014;21:342–5. https://doi.org/10.1016/j.intimp.2014.05.027.
Wang HY, Peng S, Ye Z, Li P, Li Q, Shi X, et al. Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension. Front Med. 2022;16:102–10. https://doi.org/10.1007/s11684-021-0850-9.
SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1731–4. https://doi.org/10.15585/mmwr.mm7050e1
Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Vespignani A, Dean NE. Rapid review and meta-analysis of serial intervals for SARS-CoV-2 Delta and Omicron variants. BMC Infect Dis. 2023;23:429. https://doi.org/10.1186/s12879-023-08407-5.
Puskarich MA, Cummins NW, Ingraham NE, Wacker DA, Reilkoff RA, Driver BE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957. https://doi.org/10.1016/j.eclinm.2021.100957.
Soria ME, Cortón M, Martínez-González B, Lobo-Vega R, Vázquez-Sirvent L, López-Rodríguez R, et al. High SARS-CoV-2 viral load is associated with a worse clinical outcome of COVID-19 disease. Access Microbiol. 2021;3:000259. https://doi.org/10.1099/acmi.0.000259.
China NHCotPsRo. Guideline on diagnosis and treatment of COVID-19. 2022. http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88/files/ef09aa4070244620b010951b088b8a27.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9. https://doi.org/10.1037/0033-2909.112.1.155.
Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004-18: findings from six rounds of a national survey. BMJ. 2023;380:e071952. https://doi.org/10.1136/bmj-2022-071952.
Shah H, Khan MSH, Dhurandhar NV, Hegde V. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 2021;58:831–43. https://doi.org/10.1007/s00592-020-01636-z.
Böhm M, Frey N, Giannitsis E, Sliwa K, Zeiher AM. Coronavirus Disease 2019 (COVID-19) and its implications for cardiovascular care: expert document from the German Cardiac Society and the World Heart Federation. Clin Res Cardiol. 2020;109:1446–59. https://doi.org/10.1007/s00392-020-01656-3.
Sattler A, Angermair S, Stockmann H, Heim KM, Khadzhynov D, Treskatsch S, et al. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Investig. 2020;130:6477–89. https://doi.org/10.1172/jci140965.
Gasmi A, Peana M, Pivina L, Srinath S, Gasmi Benahmed A, Semenova Y, et al. Interrelations between COVID-19 and other disorders. Clin Immunol. 2021;224:108651. https://doi.org/10.1016/j.clim.2020.108651.
Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis. 2021;21:200. https://doi.org/10.1186/s12879-021-05915-0.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–8. https://doi.org/10.1007/s00392-020-01626-9.
Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;33:944–8. https://doi.org/10.1093/ajh/hpaa096.
Lebek S, Tafelmeier M, Messmann R, Provaznik Z, Schmid C, Maier LS, et al. Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment and haemodynamic factors are associated with increased cardiac mRNA expression of angiotensin-converting enzyme 2 in patients with cardiovascular disease. Eur J Heart Fail. 2020;22:2248–57. https://doi.org/10.1002/ejhf.2020.
Li C, Yu D, Wu X, Liang H, Zhou Z, Xie Y, et al. Twelve-month specific IgG response to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. Nat Commun. 2021;12:4144. https://doi.org/10.1038/s41467-021-24230-5.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–10. https://doi.org/10.1161/CIRCULATIONAHA.104.510461.
Rao S, Lau A, So HC. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;43:1416–26. https://doi.org/10.2337/dc20-0643.
Aparisi Á, Catalá P, Amat-Santos IJ, Marcos-Mangas M, López-Otero D, Veras C, et al. Chronic use of renin-angiotensin-aldosterone inhibitors in hypertensive COVID-19 patients: results from a Spanish registry and meta-analysis. Med Clin. 2022;158:315–23. https://doi.org/10.1016/j.medcli.2021.04.005.
Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81. https://doi.org/10.1161/CIRCRESAHA.120.317134.
Pan W, Zhang J, Wang M, Ye J, Xu Y, Shen B, et al. Clinical features of COVID-19 in patients with essential hypertension and the impacts of renin-angiotensin-aldosterone system inhibitors on the prognosis of COVID-19 patients. Hypertension. 2020;76:732–41. https://doi.org/10.1161/HYPERTENSIONAHA.120.15289.
Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl J Med. 2020;382:2441–8. https://doi.org/10.1056/NEJMoa2008975.
Loader J, Taylor FC, Lampa E, Sundström J. Renin-angiotensin aldosterone system inhibitors and COVID-19: a systematic review and meta-analysis revealing critical bias across a body of observational research. J Am Heart Assoc. 2022;11:e025289. https://doi.org/10.1161/jaha.122.025289.
Yin J, Wang C, Song X, Li X, Miao M. Effects of renin-angiotensin system inhibitors on mortality and disease severity of COVID-19 patients: a meta-analysis of randomized controlled trials. Am J Hypertens. 2022;35:462–9. https://doi.org/10.1093/ajh/hpac001.
Bae JH, Choi SK, Kim NH, Lee J, Kim SG. Use of renin-angiotensin-aldosterone system inhibitors and severe COVID-19 outcomes in patients with hypertension: a nationwide cohort study. Diabetes Metab J. 2021;45:430–8. https://doi.org/10.4093/dmj.2020.0279.
Tsampasian V, Corballis N, Vassiliou VS. Renin-angiotensin-aldosterone inhibitors and COVID-19 Infection. Curr Hypertens Rep. 2022;24:425–33. https://doi.org/10.1007/s11906-022-01207-3.
Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–5. https://doi.org/10.1161/HYPERTENSIONAHA.120.15082.
Wu L, Baylan U, van der Leeden B, Schurink B, Roos E, Schalkwijk CG, et al. Cardiac inflammation and microvascular procoagulant changes are decreased in second wave compared to first wave deceased COVID-19 patients. Int J Cardiol. 2022;349:157–65. https://doi.org/10.1016/j.ijcard.2021.11.079.
Hanson PJ, Liu-Fei F, Ng C, Minato TA, Lai C, Hossain AR, et al. Correction to: characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab Investig 2022;102:1162. https://doi.org/10.1038/s41374-022-00799-3.
Liu X, Liu Y, Chen K, Yan S, Bai X, Li J, et al. Efficacy of ACEIs/ARBs vs CCBs on the progression of COVID-19 patients with hypertension in Wuhan: a hospital-based retrospective cohort study. J Med Virol. 2021;93:854–62. https://doi.org/10.1002/jmv.26315.
Acknowledgements
Thanks to the doctors and nurses in the anti-epidemic front line who participated in the data collection.
Funding
This study has received funding from Boqing Innovation Fund of the First Affiliated Hospital of Army Military Medical University (grant No. 2024BQCXJJ-8), Emergency Project for Technological Breakthrough in Clinical Treatment of Hospital-acquired COVID-19 Infection in 2023(2023XGIIT07), and Young and Middle-aged Medical Talents Foundation Project of Chongqing (Grant No. 414Z395).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Author name spelling has been corrected. In the article “Effect of the renin–angiotensin–aldosterone system inhibitors on time to nucleic acid negative conversion in hypertensive patients with SARS-CoV-2 omicron infection: a propensity score matching study” by Ru Wen et al., published in Hypertension Research (2025, 48:273–283), the name of the second author was incorrectly spelled as “Jinwen Li.” The correct spelling is “Jingwen Li.” The authors apologize for this error. The original article has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, R., Li, J., Chen, F. et al. Effect of the renin–angiotensin–aldosterone system inhibitors on time to nucleic acid negative conversion in hypertensive patients with SARS-CoV-2 omicron infection: a propensity score matching study. Hypertens Res 48, 273–283 (2025). https://doi.org/10.1038/s41440-024-01953-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01953-8
Keywords
This article is cited by
-
Impact of SARS-CoV-2 variants on viral infectivity and the role of the renin-angiotensin-aldosterone system
Hypertension Research (2025)