Abstract
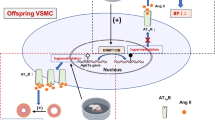
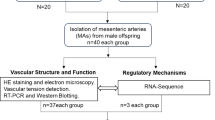
Maternal exercise during pregnancy is widely recognized as an effective means of promoting cardiovascular health in offspring. Few studies have explored how maternal exercise impacts vascular function and phenotypic switching in hypertensive offspring, despite the known involvement of vascular structural and functional remodeling in hypertension pathogenesis. Research indicates a significant relationship between elevated blood pressure and fibroblast growth factor 21 (FGF21) levels. It remains unclear whether maternal exercise during pregnancy can improve vascular function in hypertensive offspring by regulating FGF21 and its underlying mechanisms. In this study, pregnant spontaneously hypertensive rats and Wistar-Kyoto rats were randomly assigned to either a sedentary or exercise group. The exercise group underwent weightless swimming exercise from gestation day 1 (GD1) to GD20. The aim was to investigate the epigenetic modifications mediated by histone deacetylase sirtuin 1 (SIRT1) during the fetal period and the phenotypic changes in the mesenteric arteries (MAs) of hypertensive offspring. We found that maternal exercise significantly improved vascular remodeling in hypertensive offspring. Specifically, maternal exercise upregulated SIRT1 expression, which led to decreased H3K9ac (histone H3 lysine 9 acetylation) in the promoter region of the FGF21 gene. This epigenetic modification resulted in the transcriptional downregulation of FGF21 in the MAs of hypertensive fetuses. These results suggest that maternal exercise may lower blood pressure in hypertensive offspring by regulating deacetylation of the FGF21 gene promoter region through SIRT1, thereby reversing phenotypic switching and vascular structural remodeling.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ma S, Yang L, Zhao M, Magnussen CG, Xi B. Trends in hypertension prevalence, awareness, treatment and control rates among Chinese adults, 1991-2015. J Hypertens. 2021;39:740–48.
Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev. 2016;68:476–532.
Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–8.
Wang W, Wang Y, Piao H, Li B, Zhu Z, Li D, et al. Bioinformatics analysis reveals microRNA-193a-3p regulates ACTG2 to control phenotype switch in human vascular smooth muscle cells. Front Genet. 2020;11:572707.
Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem. 2019;75:229–40.
Pan X, Shao Y, Wu F, Wang Y, Xiong R, Zheng J, et al. FGF21 prevents angiotensin II-induced hypertension and vascular dysfunction by activation of ACE2/angiotensin-(1-7) axis in mice. Cell Metab. 2018;27:1323–37.
Tao K, Li M, Gu X, Wang M, Qian T, Hu L, et al. Activating transcription factor 4 aggravates angiotensin II-induced cell dysfunction in human vascular aortic smooth muscle cells via transcriptionally activating fibroblast growth factor 21. Korean J Physiol Pharmacol. 2022;26:347–55.
Kusuyama J, Alves-Wagner AB, Conlin RH, Makarewicz NS, Albertson BG, Prince NB, et al. Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab. 2021;33:939–56.
Son JS, Zhao L, Chen Y, Chen K, Chae SA, de Avila JM, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020;6:eaaz0359.
Li S, Chen Y, Zhang Y, Qiu F, Zeng F, Shi L. Prenatal exercise reprograms the development of hypertension progress and improves vascular health in SHR offspring. Vascul Pharmacol. 2021;139:106885.
Shan M, Li S, Zhang Y, Chen Y, Zhou Y, Shi L. Maternal exercise upregulates the DNA methylation of Agtr1a to enhance vascular function in offspring of hypertensive rats. Hypertens Res. 2023;46:654–66.
Zhang Y, Shan M, Ding X, Sun H, Qiu F, Shi L. Maternal exercise represses Nox4 via SIRT1 to prevent vascular oxidative stress and endothelial dysfunction in SHR offspring. Front Endocrinol. 2023;14:1219194.
Lu C, Zhao H, Liu Y, Yang Z, Yao H, Liu T, et al. Novel role of the SIRT1 in endocrine and metabolic diseases. Int J Biol Sci. 2023;19:484–501.
Wei Y-J, Wang J-F, Cheng F, Xu HJ, Chen JJ, Xiong J, et al. miR-124-3p targeted SIRT1 to regulate cell apoptosis, inflammatory response, and oxidative stress in acute myocardial infarction in rats via modulation of the FGF21/CREB/PGC1α pathway. J Physiol Biochem. 2021;77:577–87.
Stoll S, Wang C, Qiu H. DNA methylation and histone modification in hypertension. Int J Mol Sci. 2018;19:1174.
Man AWC, Li H, Xia N. The role of sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Front Physiol. 2019;10:1173.
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7.
Himuro H, Kogure M, Nakaya N, Nakamura T, Hatanaka R, Chiba I, et al. The association of birth weight and current BMI on the risk of hypertension: the Tohoku medical megabank community-based cohort study. Hypertens Res. 2024; 8. https://doi.org/10.1038/s41440-024-01827-z.
Hu X, Zhang L. Uteroplacental circulation in normal pregnancy and preeclampsia: functional adaptation and maladaptation. Int J Mol Sci. 2021;22:8622.
Barrientos G, Pussetto M, Rose M, Staff AC, Blois SM, Toblli JE. Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat. Mol Hum Reprod. 2017;23:509–19.
Chae SA, Son JS, Du M. Prenatal exercise in fetal development: a placental perspective. FEBS J. 2022;289:3058–71.
Webb AJS, Werring DJ. New insights into cerebrovascular pathophysiology and hypertension. Stroke. 2022;53:1054–64.
Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7.
Roque FR, Briones AM, García-Redondo AB, Galán M, Martínez-Revelles S, Avendaño MS, et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168:686–703.
Li G, Xu K, Xing W, Yang H, Li Y, Wang X, et al. Swimming exercise alleviates endothelial mitochondrial fragmentation via inhibiting dynamin-related protein-1 to improve vascular function in hypertension. Hypertension. 2022;9:e116–28.
Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–67.
Zhang Y, Liu D, Long X-X, Fang Q-C, Jia W-P, Li H-T. The role of FGF21 in the pathogenesis of cardiovascular disease. Chin Med J. 2021;134:2931–43.
Tan H, Yue T, Chen Z, Wu W, Xu S, Weng J. Targeting FGF21 in cardiovascular and metabolic diseases: from mechanism to medicine. Int J Biol Sci. 2023;19:66–88.
Voelter-Mahlknecht S. Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin Epigenetics. 2016;8:4.
Kusuyama J, Alves-Wagner AB, Makarewicz NS, Goodyear LJ. Effects of maternal and paternal exercise on offspring metabolism. Nat Metab. 2020;2:858–72.
Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55:71–101.
Shimizu S, Fukuda N, Chen L, Matsumoto T, Kaneda A, Endo M, et al. Abnormal epigenetic memory of mesenchymal stem and progenitor cells caused by fetal malnutrition induces hypertension and renal injury in adulthood. Hypertens Res. 2024; 26. https://doi.org/10.1038/s41440-024-01756-x.
Wang F, Chen H-Z. Histone deacetylase SIRT1, smooth muscle cell function, and vascular diseases. Front Pharmacol. 2020;11:537519.
Jing H, Lin H. Sirtuins in epigenetic regulation. Chem Rev. 2015;115:2350–75.
Gao P, Xu T-T, Lu J, Li L, Xu J, Hao DL, et al. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med. 2014;92:347–57.
Matsui S, Sasaki T, Kohno D, Yaku K, Inutsuka A, Yokota-Hashimoto H, et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat Commun. 2018;9:4604.
Lu H, Jia C, Wu D, Jin H, Lin Z, Pan J, et al. Fibroblast growth factor 21 (FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021;12:865.
Gu Y, Pais G, Becker V, Körbel C, Ampofo E, Ebert E, et al. Suppression of endothelial miR-22 mediates non-small cell lung cancer cell-induced angiogenesis. Mol Ther Nucleic Acids. 2021;26:849–64.
Yu Y, Zhang XH, Ebersole B, Ribnicky D, Wang ZQ. Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in mice. Sci Rep. 2013;3:3142.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32371183, 32200941, and 32071174), the National Key Research and Development Program of China (2022YFC3600201), and the Fundamental Research Funds for the Central Universities (2024JCYJ001 and 2024YJSY002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shan, M., Qiu, F., Li, P. et al. Maternal exercise represses FGF21 via SIRT1 to improve the phenotypic transformation of vascular smooth muscle in hypertensive offspring. Hypertens Res 48, 353–365 (2025). https://doi.org/10.1038/s41440-024-01991-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01991-2