Abstract
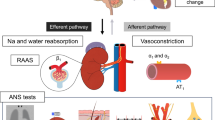
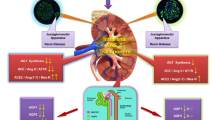
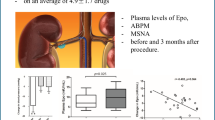
Hypertension is a common condition in cardiovascular medicine, and can lead to atrial enlargement, atrial fibrosis, and even the development of atrial fibrillation. Renal denervation (RDN) causes reduction in blood pressure (BP), but its effects on hypertension-related atrial remodeling remain unclear. This study aimed to explore the effects of RDN at BP-elevation/reduction sites guided by renal nerve stimulation (RNS) on atrial neural and structural remodeling in a hypertensive canine model. The twenty-four Chinese Kunming dogs were divided into three groups: (1) the reduced BP response ablation group (RRA group, n = 8), (2) the renal stimulation control group (RSC group, n = 8), and (3) the elevated BP response ablation group (ERA group, n = 8), which were followed for 4 weeks. Our results showed that in terms of atrial neural remodeling, compared with the RSC group, the ERA group exhibited reduced tyrosine hydroxylase (TH) protein expression and a lower TH/ choline acetyltransferase (ChAT) ratio. In contrast, the RRA group showed lower ChAT and muscarinic acetylcholine receptor 2 (CM2) protein expression, and an elevated TH/ChAT ratio. Compared with the RSC group, the ERA group presented a smaller myocyte area, reduced collagen I protein expression, and lower myocardial interstitial collagen fiber content. In contrast, the RRA group presented a larger myocyte area, increased collagen I protein expression, and greater collagen fiber content. Overall, RDN at elevated BP response sites improved atrial neural and structural remodeling under hypertensive conditions, whereas RDN at reduced BP response sites exacerbated the imbalance between atrial sympathetic and parasympathetic nerve activity and worsened structural remodeling.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–72.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:1269–324.
DiBona GF, Esler M. Translational medicine: The antihypertensive effect of renal denervation. Am J Physiol Regulatory, Integr Comp Physiol 2010;298:R245–53.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–9.
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: Basic and clinical evidence. Hypertens Res. 2022;45:198–209.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N. Engl J Med. 2014;370:1393–401.
Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the symplicity htn-3 trial. Eur Heart J. 2015;36:219–27.
Gal P, de Jong MR, Smit JJ, Adiyaman A, Staessen JA, Elvan A. Blood pressure response to renal nerve stimulation in patients undergoing renal denervation: A feasibility study. J Hum Hypertens. 2015;29:292–5.
de Jong MR, Adiyaman A, Gal P, Smit JJ, Delnoy PP, Heeg JE, et al. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension. 2016;68:707–14.
de Jong MR, Hoogerwaard AF, Adiyaman A, Smit JJJ, Heeg JE, van Hasselt B, et al. Renal nerve stimulation identifies aorticorenal innervation and prevents inadvertent ablation of vagal nerves during renal denervation. Blood Press. 2018;27:271–9.
Hoogerwaard AF, de Jong MR, Elvan A. Renal nerve stimulation as procedural end point for renal sympathetic denervation. Curr Hypertens Rep. 2018;20:24.
Zhou H, Li Y, Xu Y, Liu H, Lai Y, Tan K, et al. Mapping renal innervations by renal nerve stimulation and characterizations of blood pressure response patterns. J Cardiovasc Transl Res. 2022;15:29–37.
Tan K, Lai Y, Chen W, Liu H, Xu Y, Li Y, et al. Selective renal denervation guided by renal nerve stimulation: Mapping renal nerves for unmet clinical needs. J Hum Hypertens. 2019;33:716–24.
Liu H, Li Y, Zhou H, Chen W, Xu Y, Du H, et al. Renal nerve stimulation identifies renal innervation and optimizes the strategy for renal denervation in canine. J Transl Med. 2023;21:100.
Ripplinger CM, Noujaim SF, Linz D. The nervous heart. Prog Biophys Mol Biol. 2016;120:199–209.
Rysevaite K, Saburkina I, Pauziene N, Noujaim SF, Jalife J, Pauza DH. Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm. 2011;8:448–54.
Gottlieb LA, Mahfoud F, Stavrakis S, Jespersen T, Linz D. Autonomic nervous system: A therapeutic target for cardiac end-organ damage in hypertension. Hypertension. 2024;81:2027–37.
Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–71.
Koura T, Hara M, Takeuchi S, Ota K, Okada Y, Miyoshi S, et al. Anisotropic conduction properties in canine atria analyzed by high-resolution optical mapping: Preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation. 2002;105:2092–8.
Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, et al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. 2003;107:2051–8.
Lu J, Ling Z, Chen W, Du H, Xu Y, Fan J, et al. Effects of renal sympathetic denervation using saline-irrigated radiofrequency ablation catheter on the activity of the renin-angiotensin system and endothelin-1. J Renin-Angiotensin-Aldosterone Syst. 2014;15:532–9.
Lu J, Wang Z, Zhou T, Chen S, Chen W, Du H, et al. Selective proximal renal denervation guided by autonomic responses evoked via high-frequency stimulation in a preclinical canine model. Circ Cardiovasc Inter. 2015;8:e001847.
Chen W, Du H, Lu J, Ling Z, Long Y, Xu Y, et al. Renal artery vasodilation may be an indicator of successful sympathetic nerve damage during renal denervation procedure. Sci Rep. 2016;6:37218.
Chen WJ, Liu H, Wang ZH, Liu C, Fan JQ, Wang ZL, et al. The impact of renal denervation on the progression of heart failure in a canine model induced by right ventricular rapid pacing. Front Physiol. 2019;10:1625.
Liu H, Chen W, Lai Y, Du H, Wang Z, Xu Y, et al. Selective renal denervation guided by renal nerve stimulation in canine. Hypertension. 2019;74:536–45.
Lai Y, Zhou H, Chen W, Liu H, Liu G, Xu Y, et al. The intrarenal blood pressure modulation system is differentially altered after renal denervation guided by different intensities of blood pressure responses. Hypertension Res. 2022;46:456–67.
Schneider CA, Rasband WS, Eliceiri KW. Nih image to imagej: 25 years of image analysis. Nat methods. 2012;9:671–5.
Govindasamy L, Pedersen B, Lian W, Kukar T, Gu Y, Jin S, et al. Structural insights and functional implications of choline acetyltransferase. J Struct Biol. 2004;148:226–35.
Kakinuma Y, Akiyama T, Sato T. Cholinoceptive and cholinergic properties of cardiomyocytes involving an amplification mechanism for vagal efferent effects in sparsely innervated ventricular myocardium. FEBS J. 2009;276:5111–25.
Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–51.
Chester AH, McCormack A, Miller EJ, Ahmed MN, Yacoub MH. Coronary vasodilation mediated by T cells expressing choline acetyltransferase. Am J Physiol Heart Circ Physiol. 2021;321:H933–9.
Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharm Res. 2001;44:161–82.
Mompeo B, Maranillo E, Garcia-Touchard A, Larkin T, Sanudo J. The gross anatomy of the renal sympathetic nerves revisited. Clin Anat. 2016;29:660–4.
van Amsterdam WAC, Blankestijn PJ, Goldschmeding R, Bleys RLAW. The morphological substrate for renal denervation: Nerve distribution patterns and parasympathetic nerves. A post-mortem histological study. Ann Anat - Anatomischer Anz 2016;204:71–9.
Cheng X, Zhang Y, Chen R, Qian S, Lv H, Liu X, et al. Anatomical evidence for parasympathetic innervation of the renal vasculature and pelvis. J Am Soc Nephrol. 2022;33:2194–210.
Kiuchi MG, Esler MD, Fink GD, Osborn JW, Banek CT, Böhm M, et al. Renal denervation update from the international sympathetic nervous System Summit: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:3006–17.
Kemp DR. A histological and functional study of the gastric mucosal innervation in the dog. Part I; The quantification of the fibre content of the normal supradiphragmatic vagal trunks and their abdominal branches. Aust N. Z J Surg. 1973;43:288–94.
Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. acc/aha/accp/hrs guideline for the diagnosis and management of atrial fibrillation: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2023;2024:e1–e156.
Lauder L, Mahfoud F, Azizi M, Bhatt DL, Ewen S, Kario K, et al. Hypertension management in patients with cardiovascular comorbidities. Eur Heart J. 2023;44:2066–77.
Hanna P, Buch E, Stavrakis S, Meyer C, Tompkins JD, Ardell JL, et al. Neuroscientific therapies for atrial fibrillation. Cardiovasc Res. 2021;117:1732–45.
Sohinki D, Stavrakis S. New approaches for treating atrial fibrillation: Focus on autonomic modulation. Trends Cardiovasc Med. 2020;30:433–9.
Zhao J, Zhang Y, Yin Z, Zhu Y, Xin F, Zhang H, et al. Impact of proinflammatory epicardial adipose tissue and differentially enhanced autonomic remodeling on human atrial fibrillation. J Thorac Cardiovasc Surg. 2023;165:e158–74.
Verheule S, Sato T, Everett T 4th, Engle SK, Otten D, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of tgf-beta1. Circ Res. 2004;94:1458–65.
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70.
Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: The eradicate-af randomized clinical trial. JAMA. 2020;323:248–55.
Acknowledgements
We would like to express our gratitude to American Journal Experts for their invaluable assistance in the language editing of this manuscript. This study was supported by the National Natural Science Foundation of China (Grant Numbers: 32071110 and Yin Yue-Hui) and the Natural Science Foundation of Chongqing, China (No. CSTB2024NSCQ-MSX0595 Chen Wei-Jie).
Author information
Authors and Affiliations
Contributions
DL contributed to conceptualization, methodology, software development, formal analysis, investigation, data curation, original draft preparation, and review and editing. YL, HZ, WC, YL, YR, and XM contributed to the methodology, formal analysis, investigation, data curation, visualization, review and editing. HK, XH, GL, and ZL contributed resources, data curation, review and editing, and visualization. PX and YY contributed to conceptualization, methodology, validation, review and editing, supervision, and project administration. All the authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Animal Experiment Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University, following the guidelines of the National Institutes of Health for the care and use of laboratory animals, and standard experimental animal care was provided at the Animal Experiment Center of Chongqing Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dan Li, Long, Y., Zhou, H. et al. Effects of renal denervation at BP-elevation/reduction sites guided by renal nerve stimulation on atrial neural and structural remodeling in a hypertensive canine model. Hypertens Res 48, 2387–2400 (2025). https://doi.org/10.1038/s41440-025-02258-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02258-0