Abstract
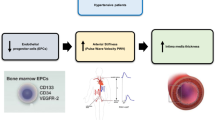
Neutrophil extracellular traps (NETs) are increasingly implicated in the pathophysiology of cardiovascular disease; however, data regarding their clinical significance in hypertension-mediated vascular damage is scarce. We recruited untreated, newly diagnosed patients with essential hypertension (UHTs) and age- and sex- matched healthy, normotensive controls (NTs). Ambulatory blood pressure (BP) monitoring was performed with the Mobil-O-Graph-NG device. NET-specific myeloperoxidase (MPO)-DNA complexes were measured in plasma with ELISA. Carotid pulse wave velocity (PWV) was calculated by using a high-definition echo-tracking system. Skin microcirculation was assessed using laser speckle contrast imaging coupled with post-occlusive reactive hyperemia. A total of 139 participants of whom 99 UHTs (mean age 48.9 ± 8.34 years) and 40 NTs (mean age 47.4 ± 7.38 years) were included in the study. Using categorical regression with optimal scaling, various peripheral and central BP parameters were significantly associated with MPO-DNA complexes levels; 24 h peripheral systolic BP (p < 0.001) was the strongest predictor of hypertension-related NETosis, after adjusting for potential confounders. MPO-DNA complexes levels were positively associated with mean carotid PWV (p = 0.033) and baseline flux (p = 0.017) and negatively associated with baseline to peak flux (p = 0.020) and cutaneous vascular conductance increase (p = 0.034). In multivariate analysis, carotid PWV showed an independent association with MPO-DNA complexes. Circulating NETs levels are tightly associated with BP. Of note, the 24 h peripheral SBP load is the strongest predictor of NETs burden. Moreover, NETs are strongly related to surrogate measures of micro- and macrovascular damage, suggesting their potential role as a diagnostic and/or therapeutic target in hypertension.

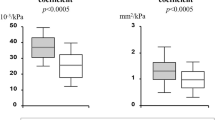
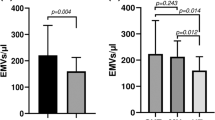
MPO-DNA levels are strongly associated with peripheral and central BP parameters in untreated hypertensives, with 24h peripheral SBP being the strongest predictor of circulating NETs burden. MPO-DNA levels are correlated with surrogate markers of micro- and macrovascular damage. MPO-DNA levels are independently associated with carotid artery stiffness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertensi. J Hypertens. 2023;41:1874–2071.
Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130:466–74.
Staplin N, de la Sierra A, Ruilope LM, Emberson JR, Vinyoles E, Gorostidi M, et al. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59124 patients. Lancet. 2023;401:2041–50.
Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900.
Lieb W, Enserro DM, Sullivan LM, Vasan RS. Residual cardiovascular risk in individuals on blood pressure-lowering treatment. J Am Heart Assoc. 2015;4:1–10.
Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:719.
Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517–32.
Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. 2020;108:377–96.
Boeltz S, Amini P, Anders HJ, Andrade F, Bilyy R, Chatfield S, et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36:1405–14.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–43.
Thakur M, Junho CVC, Bernhard SM, Schindewolf M, Noels H, Döring Y. NETs-Induced thrombosis impacts on cardiovascular and chronic kidney disease. Circ Res. 2023;132:933–49.
Krishnan J, Hennen EM, Ao M, Kirabo A, Ahmad T, de la Visitación N, et al. NETosis drives blood pressure elevation and vascular dysfunction in hypertension. Circ Res. 2024;134:1483–94.
Chrysanthopoulou A, Gkaliagkousi E, Lazaridis A, Arelaki S, Pateinakis P, Ntinopoulou M, et al. Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. JCI Insight. 2021;6:e148668.
Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–71.
Yang S, De-Zhao W, Hong-Xia Z, He W, Chen X. Echo-tracking technology assessment of carotid artery stiffness in patients with coronary slow flow. Ultrasound Med Biol. 2015;41:72–6.
Vriz O, Aboyans V, Minisini R, Magne J, Bertin N, Pirisi M, et al. Reference values of one-point carotid stiffness parameters determined by carotid echo-tracking and brachial pulse pressure in a large population of healthy subjects. Hypertens Res. 2017;40:685–95.
Antonini-Canterin F, Roşca M, Beladan CC, Popescu BA, Piazza R, Leiballi E, et al. Echo-tracking assessment of carotid artery stiffness in patients with aortic valve stenosis. Echocardiography. 2009;26:823–31.
Lazaridis A, Triantafyllou A, Dipla K, Dolgyras P, Koletsos N, Anyfanti P, et al. Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens Res. 2022;45:445–54.
Draijer M, Hondebrink E, Van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 2009;24:639–51.
Senarathna J, Member S, Rege A, Li N, Thakor NV. Laser Speckle Contrast Imaging: Theory, Instrumentation and Applications. IEEE Rev Biomed Eng. 2013;6:99–110.
Hidalgo A, Libby P, Soehnlein O, Aramburu IV, Papayannopoulos V, Silvestre-Roig C. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc Res. 2022;118:2737–53.
Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9:1–17.
Borissoff J, Joosen I, Versteylen M, Brill A, Fuchs T, Savchenko A, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arter Thromb Vasc Biol. 2013;33:2032–40.
Stakos D, Skendros P, Konstantinides S, Ritis K. Traps N’ Clots: NET-Mediated Thrombosis and Related Diseases. Thromb Haemost. 2020;120:373–83.
Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–8.
Mulè G, Nardi E, Andronico G, Cottone S, Raspanti F, Piazza G, et al. Relationships between 24 h blood pressure load and target organ damage in patients with mild-to-moderate essential hypertension. Blood Press Monit. 2001;6:115–23.
de la Sierra A, Pareja J, Fernández-Llama P, Armario P, Yun S, Acosta E, et al. Twenty-four-hour central blood pressure is not better associated with hypertensive target organ damage than 24-h peripheral blood pressure. J Hypertens. 2017;35:2000–5.
Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Staessen JA, et al. Association of target organ damage with 24-hour systolic and diastolic blood pressure levels and hypertension subtypes in untreated Chinese. Hypertension. 2014;63:222–8.
Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, et al. Risk factors associated with alterations in carotid intima-media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. J Hypertens. 1998;16:949–61.
Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group Study Ambul Monit Blood Press Lisinopril Evaluation Circulation. 1997;95:1464–70.
Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–15.
Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: A population based study. Am J Hypertens. 2006;19:243–50.
Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, De Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–46.
Staessen JA, Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA J Am Med Assoc. 2019;322:409–20.
McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. 2024;45:3912–4018.
Antonios TFT, Singer DRJ, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–8.
Qi H, Yang S, Zhang L. Endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol. 2017;8:928.
Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16.
Martinod K, Wagner DD. Thrombosis: Tangled up in NETs. Blood. 2014;123:2768–76.
Acknowledgements
Part of this work was funded by the Hellenic Society of Hypertension and Greek General Secretariat for Research and Innovation (GSRI), Regional Excellence Programme InTechThrace, grant MIS-5047285.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed equally to the manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malliora, A., Lazaridis, A., Natsi, AM. et al. Increasing blood pressure predicts levels of circulating neutrophil extracellular traps in untreated hypertensive patients. Hypertens Res 48, 2688–2700 (2025). https://doi.org/10.1038/s41440-025-02306-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02306-9