Abstract
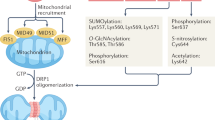
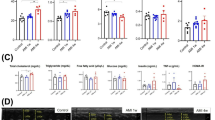
Mitochondria are dynamic organelles that can change their morphology. The role of these mitochondrial dynamics in cardiomyocytes remains obscure in patients with heart failure (HF). Endomyocardial biopsies were performed consecutively in 127 HF patients, and mitochondrial morphology data were obtained from 111 patients by electron microscopy. The patients were divided into three groups according to mitochondrial area quartiles (fission [Q1, area ≤ 0.119 μm2, n = 27], normal [Q2/Q3, 0.120 μm2 ≤ area ≤ 0.178 μm2, n = 55], and fusion [Q4, area ≥ 0.179 μm2, n = 28]). In the fission group, the serum N-terminal pro-brain natriuretic peptide and B-type natriuretic peptide (BNP) levels were significantly higher, and patients with HF and a reduced left ventricular ejection fraction were more common, than in the other groups. A multivariate logistic regression model showed that diabetes mellitus was independently associated with placement in the fission group (odds ratio: 2.835, 95%confidence interval [CI]: 1.037–7.752). A Kaplan–Meier curve analysis showed that the prognosis was significantly poorer in the fission group than in the other groups, and a multivariate Cox regression model revealed fission to be an independent predictor of 1500-day mortality (hazard ratio: 4.365, 95%CI: 1.198–15.909). The circulating levels of miR-140-5p (≥2500) were independently associated with the presence of mitochondrial fission (OR: 3.622, 95%CI: 1.260–10.413). Excessive mitochondrial fission was observed in patients with severe HF status, and was independently associated with adverse outcomes in HF patients. Circulating mitochondrial dynamics-related miRNA levels might be of use in detecting mitochondrial fission in the cardiomyocytes of HF patients.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The deidentified participant data will not be shared.
References
Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J. 2013;77:2209–17.
Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–92. https://doi.org/10.1002/ejhf.319.
Shirakabe A, Asai K, Hata N, Yokoyama S, Shinada T, Kobayashi N, et al. Clinical significance of matrix metalloproteinase (MMP)-2 in patients with acute heart failure. Int Heart J. 2010;51:404–10. https://doi.org/10.1536/ihj.51.404.
Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, et al. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J. 2013;77:687–96. https://doi.org/10.1253/circj.cj-12-0994.
Shirakabe A, Okazaki H, Matsushita M, Shibata Y, Shigihara S, Nishigoori S, et al. Type III procollagen peptide level can indicate liver dysfunction associated with volume overload in acute heart failure. ESC Heart Fail. 2022;9:1832–43. https://doi.org/10.1002/ehf2.13878.
Sawatani T, Shirakabe A, Okazaki H, Matsushita M, Shibata Y, Shigihara S, et al. Clinical significance of the N-terminal pro-brain natriuretic peptide and B-type natriuretic peptide ratio in the acute phase of acute heart failure. Eur Heart J Acute Cardiovasc Care. 2021;10:1016–26. https://doi.org/10.1093/ehjacc/zuab068.
Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–78. https://doi.org/10.1161/CIRCRESAHA.116.303356.
Ikeda Y, Shirakabe A, Brady C, Zablocki D, Ohishi M, Sadoshima J. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol. 2015;78:116–22. https://doi.org/10.1016/j.yjmcc.2014.09.019.
Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, et al. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid Med Cell Longev. 2014;2014:210934. https://doi.org/10.1155/2014/210934.
Iwatani N, Kubota K, Ikeda Y, Tokushige A, Miyanaga S, Higo K, et al. Different characteristics of mitochondrial dynamics-related miRNAs on the hemodynamics of pulmonary artery hypertension and chronic thromboembolic pulmonary hypertension. J Cardiol. 2021;78:24–30. https://doi.org/10.1016/j.jjcc.2021.03.008.
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure- Digest Version. Circ J. 2019;83:2084–184. https://doi.org/10.1253/circj.CJ-19-0342.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. https://doi.org/10.1002/ejhf.592.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Sasaki Y, Ikeda Y, Uchikado Y, Akasaki Y, Sadoshima J, Ohishi M. Estrogen plays a crucial role in Rab9-dependent mitochondrial autophagy, delaying arterial senescence. J Am Heart Assoc. 2021;10:e019310. https://doi.org/10.1161/JAHA.120.019310.
Gu D, Zou X, Ju G, Zhang G, Bao E, Zhu Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016;2016:2093940. https://doi.org/10.1155/2016/2093940.
Li J, Li Y, Jiao J, Wang J, Qin D, Li P. Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol. 2014;34:1788–99. https://doi.org/10.1128/MCB.00774-13.
Zhao Y, Ponnusamy M, Liu C, Tian J, Dong Y, Gao J, et al. MiR-485-5p modulates mitochondrial fission through targeting mitochondrial anchored protein ligase in cardiac hypertrophy. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2871–81. https://doi.org/10.1016/j.bbadis.2017.07.034.
Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. https://doi.org/10.1038/nm.2282.
Uchikado Y, Ikeda Y, Ohishi M. Current understanding of the pivotal role of mitochondrial dynamics in cardiovascular diseases and senescence. Front Cardiovasc Med. 2022;9:905072. https://doi.org/10.3389/fcvm.2022.905072.
Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation. 2016;133:1249–63. https://doi.org/10.1161/CIRCULATIONAHA.115.020502.
Din S, Mason M, Völkers M, Johnson B, Cottage CT, Wang Z, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci USA 2013;110:5969–74. https://doi.org/10.1073/pnas.1213294110.
Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–97. https://doi.org/10.1161/CIRCRESAHA.111.263848.
Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–14. https://doi.org/10.1089/ars.2012.4777.
Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–57. https://doi.org/10.1089/ars.2010.3286.
Iheagwam FN, Joseph AJ, Adedoyin ED, Iheagwam OT, Ejoh SA. Mitochondrial Dysfunction in Diabetes: Shedding Light on a Widespread Oversight. Pathophysiology 2025;32. https://doi.org/10.3390/pathophysiology32010009.
Wei L, Fang C, Jiang Y, Zhang H, Gao P, Zhou X, et al. The role of placental MFF-mediated mitochondrial fission in gestational Diabetes Mellitus. Diabetes Metab Syndr Obes. 2025;18:541–54. https://doi.org/10.2147/DMSO.S484002.
Abercrombie DM, Kanmera T, Angal S, Tamaoki H, Chaiken IM. Cooperative interactions in neurophysin-neuropeptide hormone complexes. Analytical affinity chromatography of native and covalently-modified neurophysins. Int J Pept Protein Res. 1984;24:218–32.
Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin Endocrinol. 1997;47:287–96. https://doi.org/10.1046/j.1365-2265.1997.2361058.x.
Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–9. https://doi.org/10.1161/CIRCULATIONAHA.109.924167.
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28:3076–93. https://doi.org/10.1093/eurheartj/ehm456.
Acknowledgements
We thank the staff of Kagoshima University and Nippon Medical School Chiba Hokusoh Hospital for collecting the medical data. We would like to thank Uni-edit (https://uni-edit.net/) for editing and proofreading this manuscript.
Funding
This research received Grants-in-Aid for Scientific Research grants from the Ministry of Health, Labor and Welfare in Japan. This research received no grants from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirakabe, A., Ikeda, Y., Uchikado, Y. et al. Prognostic impact of excessive mitochondrial fission in patients with heart failure and evaluation of mitochondrial dynamics-related miRNAs in heart failure. Hypertens Res (2025). https://doi.org/10.1038/s41440-025-02338-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41440-025-02338-1