Abstract
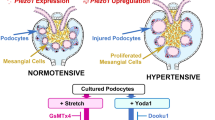
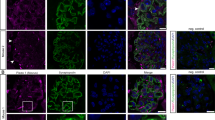
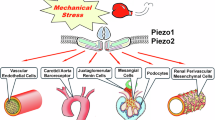
Piezo1 is a mechanosensitive ion channel that mediates a broad range of biological and pathological phenomena in living organisms, including hypertension and hypertensive nephropathy. We previously reported the upregulation of Piezo1 in the glomerular podocytes of hypertensive nephropathy mice in vivo and a mechanical stretch-induced podocyte injury cascade via Piezo1 and Rac1 in vitro, suggesting the pathogenic involvement of Piezo1. However, recent reports on podocyte-specific Piezo1 knockout mice with various podocyte injury models other than hypertension have yielded inconsistent results, indicating pathogenic and anti-pathogenic roles of Piezo1. In this study, we generated podocyte-specific Piezo1 knockout mice and examined their podocyte phenotypes under normotensive and hypertensive conditions induced by angiotensin II and a high-salt diet. Podocyte-specific Piezo1 knockout mice did not spontaneously develop podocyte injury. Angiotensin II infusion and a high-salt diet for 14 days caused hypertension, but not significant podocyte injury and proteinuria in the littermate control mice, which were strengthened by podocyte-specific Piezo1 deletion. Through comprehensive transcriptome analysis of the glomeruli of wild-type and podocyte-specific Piezo1 knockout mice, we found altered expression of several genes, including Rhpn1 encoding Rho GTPase binding protein Rhophilin1. Podocyte injury in hypertensive podocyte-specific Piezo1 knockout mice was inhibited by the angiotensin II receptor blocker losartan, the anti-hypertensive drug hydralazine, and partially ameliorated by the Rho kinase inhibitor fasudil, suggesting that podocyte injury in the knockout mice may be mediated by Rhophilin1 and/or Rho signaling downstream of Piezo1. Findings related to the hypertensive podocyte injury model support the possible anti-pathogenic protective roles of Piezo1.

We created podocyte-specific Piezo1 KO mice, and hypertension was induced by angiotensin II and a high-salt diet. Hypertension-evoked proteinuria and podocyte injury were exaggerated in the KO mice. They were ameliorated by anti-hypertensive drugs and Rho kinase inhibitor, suggesting protective roles of Piezo1 and involvement of RhoA signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
12 May 2022
“IIn xml, duplicated text in Abstract has been removed.” is to be added to the A++ of the publication.
References
Mann N, Sun H, Majmundar AJ. Mechanisms of podocyte injury in genetic kidney disease. Pediatr Nephrol. 2025;40:1523–38.
Dworkin LD, Hostetter TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest. 1984;73:1448–61.
Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–9.
Kimura G, Brenner BM The renal basis for salt sensitivity in hypertension. In: Laragh JH, Brenner BM (eds). Hypertension, pathophysiology, diagnosis, and management. 2nd ed. Raven Press: New York, NY, USA, 1995, Vol 1. 1569-88.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–93.
Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–83.
Nagase M, Fujita T. Mineralocorticoid receptor activation in obesity hypertension. Hypertens Res. 2009;32:649–57.
Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–44.
Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–4.
Endlich K, Kliewe F, Endlich N. Stressed podocytes-mechanical forces, sensors, signaling and response. Pflug Arch. 2017;469:937–49.
Reynolds PA. The mechanobiology of kidney podocytes in health and disease. Clin Sci. 2020;134:1245–53.
Haydak J, Azeloglu EU. Role of biophysics and mechanobiology in podocyte physiology. Nat Rev Nephrol. 2024;20:371–85.
Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–86.
Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, et al. Podocyte purinergic P2X4 channels are mechanotransducers that mediate cytoskeletal disorganization. J Am Soc Nephrol. 2016;27:848–62.
Tu YC, Shu HP, Sun LL, Liao QQ, Feng L, Ren M, et al. The physiopathologic roles of calcium signaling in podocytes. Front Biosci (Landmark Ed). 2023;28:240, https://doi.org/10.31083/j.fbl2810240.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60.
Earley S, Santana LF, Lederer WJ. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol Rev. 2022;102:1153–8.
Huang J, Zhang K, Du R, Liu W, Zhang H, Tian T, et al. The Janus-faced role of Piezo1 in cardiovascular health under mechanical stimulation. Genes Dis. 2023;10:1956–68.
Coste B, Delmas P. PIEZO ion channels in cardiovascular functions and diseases. Circ Res. 2024;134:572–91.
Nagase T, Nagase M. Piezo ion channels: long-sought-after mechanosensors mediating hypertension and hypertensive nephropathy. Hypertens Res. 2024;47:2786–99.
Mochida Y, Ochiai K, Nagase T, Nonomura K, Akimoto Y, Fukuhara H, et al. Piezo2 expression and its alteration by mechanical forces in mouse mesangial cells and renin-producing cells. Sci Rep. 2022;12:4197, https://doi.org/10.1038/s41598-022-07987-7.
Ochiai K, Mochida Y, Nagase T, Fukuhara H, Yamaguchi Y, Nagase M. Upregulation of Piezo2 in the mesangial, renin, and perivascular mesenchymal cells of the kidney of Dahl salt-sensitive hypertensive rats and its reversal by esaxerenone. Hypertens Res. 2023;46:1234–46.
Oba R, Ueno H, Oishi A, Nagahama K, Kanzaki G, Tsuboi N, et al. Upregulation of Piezo2 and increased extracellular matrix protein in diabetic kidney disease mice. Hypertens Res. 2025;48:1514–28.
Ogino S, Yoshikawa K, Nagase T, Mikami K, Nagase M. Roles of the mechanosensitive ion channel Piezo1 in the renal podocyte injury of experimental hypertensive nephropathy. Hypertens Res. 2024;47:747–59.
Fu R, Wang W, Huo Y, Li L, Chen R, Lin Z, et al. The mechanosensitive ion channel Piezo1 contributes to podocyte cytoskeleton remodeling and development of proteinuria in lupus nephritis. Kidney Int. 2024;106:625–39.
Li W, Zhang Z, Peng Z, Hu H, Cui X, Zhu Z, et al. Piezo1-mediated calcium signaling and podocyte injury in diabetic kidney disease. J Am Soc Nephrol. 2025;36:1310–26.
Melica ME, Antonelli G, Semeraro R, La Regina G, Dafichi T, Fantini C, et al. Piezo1, F-actin remodeling, and podocyte survival and regeneration. J Am Soc Nephrol. e-pub ahead of print 2025/04/02; https://doi.org/10.1681/asn.0000000697.
Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, et al. Piezo1 and G(q)/G(11) promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215:2655–72.
Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. eLife 2015;4:e07370, https://doi.org/10.7554/eLife.07370.
Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005;16:2257–62.
Kasahara M, Mukoyama M, Sugawara A, Makino H, Suganami T, Ogawa Y, et al. Ameliorated glomerular injury in mice overexpressing brain natriuretic peptide with renal ablation. J Am Soc Nephrol. 2000;11:1691–701.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Chung JJ, Goldstein L, Chen YJ, Lee J, Webster JD, Roose-Girma M, et al. Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury. J Am Soc Nephrol. 2020;31:2341–54.
Lal MA, Andersson AC, Katayama K, Xiao Z, Nukui M, Hultenby K, et al. Rhophilin-1 is a key regulator of the podocyte cytoskeleton and is essential for glomerular filtration. J Am Soc Nephrol. 2015;26:647–62.
Greiten JK, Kliewe F, Schnarre A, Artelt N, Schröder S, Rogge H, et al. The role of filamins in mechanically stressed podocytes. FASEB J. 2021;35:e21560, https://doi.org/10.1096/fj.202001179RR.
Kliewe F, Kaling S, Lötzsch H, Artelt N, Schindler M, Rogge H, et al. Fibronectin is up-regulated in podocytes by mechanical stress. FASEB J. 2019;33:14450–60.
Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24:193–204.
Dalghi MG, Clayton DR, Ruiz WG, Al-Bataineh MM, Satlin LM, Kleyman TR, et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am J Physiol Ren Physiol. 2019;317:F303–21.
Zhao Y, Duan J, Han ID, van de Leemput J, Ray PE, Han Z. Piezo, nephrocyte function, and slit diaphragm maintenance in Drosophila. J Am Soc Nephrol. 2025;36:393–405.
Schulz K, Hazelton-Cavill P, Alornyo KK, Edenhofer I, Lindenmeyer M, Lohr C, et al. Piezo activity levels need to be tightly regulated to maintain normal morphology and function in pericardial nephrocytes. Sci Rep. 2024;14:28254, https://doi.org/10.1038/s41598-024-79352-9.
Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, et al. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996;271:645–8.
Jin YJ, Chennupati R, Li R, Liang G, Wang S, Iring A, et al. Protein kinase N2 mediates flow-induced endothelial NOS activation and vascular tone regulation. J Clin Invest. 2021;131:e145734, https://doi.org/10.1172/JCI145734.
Acknowledgements
We are grateful to Prof. Ardem Patapoutian for his kind gift of the Piezo1fl/fl mice, to Prof. Hidetake Kurihara for providing the rat podocyte cell line, and to Prof. Yoshihiro Akimoto and Prof. Akihiko Kudo for their help with electron microscopy.
Funding
This work was supported in part by JSPS KAKENHI Grant Numbers JP17K09736, JP20K08616, JP23K07704, by Japan Agency for Medical Research and Development (18gm5810019h9903), and by the Salt Science Research Foundation, No.2329.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mikami, K., Nagase, T., Kishino, H. et al. Podocyte-specific deletion of mechanochannel Piezo1 exacerbates proteinuria and podocyte injury in mouse hypertensive nephropathy. Hypertens Res (2025). https://doi.org/10.1038/s41440-025-02383-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41440-025-02383-w