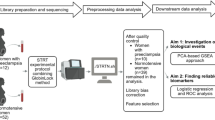
Abstract
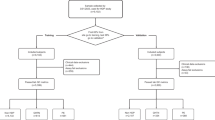
Emerging evidence links maternal immune dysregulation to hypertensive disorders of pregnancy (HDP), yet gestational immune alterations preceding symptom onset remain unclear. This study aimed to evaluate the associations between first-trimester immune biomarkers and incident HDP risk across clinical subtypes. This retrospective cohort study enrolled pregnant women aged ≥18 years undergoing first-trimester antenatal screening at a tertiary hospital from March to November 2023. First-trimester peripheral immune markers—neutrophils, monocytes, lymphocytes, and platelets—were measured, with derived indices including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), and aggregate index of systemic inflammation (AISI). Outcomes included HDP, gestational hypertension (GHTN), and preeclampsia confirmed via electronic medical records. Multivariable logistic regression models were performed to evaluate the relationship between peripheral immune markers and outcomes. Among the 2739 pregnant women who met inclusion criteria, 195 developed HDP, including 96 GHTN and 99 preeclampsia. Multivariable logistic regression demonstrated that first-trimester neutrophils, monocytes, platelets, lymphocytes, SII, and AISI were independently and positively associated with HDP risk in a linear dose-response manner (all FDR P < 0.05), with platelets exhibiting the strongest association (OR T3 vs. T1: 2.20; per log-SD: OR = 1.55). Distinct biomarker profiles were identified between GHTN and preeclampsia: GHTN exhibited associations with neutrophils, platelets, SII, and AISI, while preeclampsia correlated with monocytes, platelets, lymphocytes, SII, and AISI (all FDR P < 0.05). Elevated first-trimester immune markers correlate with HDP, particularly platelet-related indices. Divergent immune signatures between GHTN and preeclampsia suggest subtype-specific pathophysiological mechanisms.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Some or all datasets generated during or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1:e000101 https://doi.org/10.1136/bmjopen-2011-000101.
Hinkle SN, Schisterman EF, Liu D, Pollack AZ, Yeung EH, Mumford SL, et al. Pregnancy complications and long-term mortality in a diverse cohort. Circulation. 2023;147:1014–25. https://doi.org/10.1161/CIRCULATIONAHA.122.062177.
Huang C, Wei K, Lee PMY, Qin G, Yu Y, Li J. Maternal hypertensive disorder of pregnancy and mortality in offspring from birth to young adulthood: national population based cohort study. BMJ. 2022;379:e072157 https://doi.org/10.1136/bmj-2022-072157.
Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–34. https://doi.org/10.1016/j.jacc.2020.03.028.
Laresgoiti-Servitje E, Gómez-López N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16:510–24. https://doi.org/10.1093/humupd/dmq007.
Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–87. https://doi.org/10.1016/s0143-4004(03)00063-8.
Cornelius DC, Cottrell J, Amaral LM, LaMarca B. Inflammatory mediators: a causal link to hypertension during preeclampsia. Br J Pharm. 2019;176:1914–21. https://doi.org/10.1111/bph.14466.
Wang Y, Li B, Tong F. Global trends in research of immune cells associated with hypertensive disorders of pregnancy: A 20-year bibliometric analyses (from 2001 to 2021). Front Immunol. 2022;13:1036461 https://doi.org/10.3389/fimmu.2022.1036461.
Gyselaers W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am J Obstet Gynecol. 2022;226. https://doi.org/10.1016/j.ajog.2021.11.022.
Vikberg S, Lindau R, Solders M, Raffetseder J, Budhwar S, Ernerudh J, et al. Labour promotes systemic mobilisation of monocytes, T cell activation and local secretion of chemotactic factors in the intervillous space of the placenta. Front Immunol. 2023;14:1129261 https://doi.org/10.3389/fimmu.2023.1129261.
Wang F, Jia W, Fan M, Shao X, Li Z, Liu Y, et al. Single-cell immune landscape of human recurrent miscarriage. Genomics Proteom Bioinforma. 2021;19:208–22. https://doi.org/10.1016/j.gpb.2020.11.002.
Arenas-Hernandez M, Romero R, Gershater M, Tao L, Xu Y, Garcia-Flores V, et al. Specific innate immune cells uptake fetal antigen and display homeostatic phenotypes in the maternal circulation. J Leukoc Biol. 2022;111:519–38. https://doi.org/10.1002/JLB.5HI0321-179RR.
Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506.
Mohamed RA, Ali IA. Role of neutrophil/lymphocyte ratio, uric acid/albumin ratio and uric acid/creatinine ratio as predictors to severity of preeclampsia. BMC Pregnancy Childbirth. 2023;23:763. https://doi.org/10.1186/s12884-023-06083-6.
Mtali YS, Lyimo MA, Luzzatto L, Massawe SN. Hypertensive disorders of pregnancy are associated with an inflammatory state: evidence from hematological findings and cytokine levels. BMC Pregnancy Childbirth. 2019;19:237. https://doi.org/10.1186/s12884-019-2383-7.
Ishiyama S, Mochizuki K, Shinohara R, Miyake K, Kushima M, Kojima R, et al. Association of maternal leukocyte, monocyte, and neutrophil counts with hypertensive disorders of pregnancy: the Japan Environment and Children’s Study (JECS). Sci Rep. 2024;14:7248. https://doi.org/10.1038/s41598-024-55623-3.
Örgül G, Aydın Haklı D, Özten G, Fadiloğlu E, Tanacan A, Beksaç MS. First trimester complete blood cell indices in early and late onset preeclampsia. Turk J Obstet Gynecol. 2019;16:112–7. https://doi.org/10.4274/tjod.galenos.2019.93708.
Elmaradny E, Alneel G, Alkhattaf N, AlGadri T, Albriakan N. Predictive values of combined platelet count, neutrophil-lymphocyte ratio, and platelet-lymphocyte ratio in preeclampsia. J Obstet Gynaecol. 2022;42:1011–7. https://doi.org/10.1080/01443615.2021.1986476.
Woldeamanuel GG, Tlaye KG, Wu L, Poon LC, Wang CC. Platelet count in preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5:100979 https://doi.org/10.1016/j.ajogmf.2023.100979.
Al-Nuaimi AMA. Role of hematological indices in predicting preeclampsia and its severity: retrospective case-control study. Medicine. 2024;103:e38557 https://doi.org/10.1097/MD.0000000000038557.
Kim M-A, Han GH, Kwon J-Y, Kim Y-H. Clinical significance of platelet-to-lymphocyte ratio in women with preeclampsia. Am J Reprod Immunol. 2018;80:12973 https://doi.org/10.1111/aji.12973.
Wang J, Zhu Q-W, Cheng X-Y, Liu JY, Zhang LL, Tao YM, et al. Assessment efficacy of neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in preeclampsia. J Reprod Immunol. 2019;132:29–34. https://doi.org/10.1016/j.jri.2019.02.001.
Panwar M, Kumari A, Hp A, Arora R, Singh V, Bansiwal R. Raised neutrophil lymphocyte ratio and serum beta hCG level in early second trimester of pregnancy as predictors for development and severity of preeclampsia. Drug Discov Ther. 2019;13:34–7. https://doi.org/10.5582/ddt.2019.01006.
Xie S, Zhang E, Gao S, Su S, Liu J, Zhang Y, et al. Associations of systemic immune-inflammation index and systemic inflammation response index with maternal gestational diabetes mellitus: Evidence from a prospective birth cohort study. Chin Med J. 2024. https://doi.org/10.1097/CM9.0000000000003236.
Wang J, Zhang Z, Sun Y, Yu B, Wang Y, Lu Y, et al. Association of innate versus specific immunity with heart failure incidence: a prospective study. Heart. 2024;111:76–82. https://doi.org/10.1136/heartjnl-2024-324591.
Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China (2020). Zhonghua Fu Chan Ke Za Zhi. 2020;55:227–38. https://doi.org/10.3760/cma.j.cn112141-20200114-00039.
Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202:654.e1–e6. https://doi.org/10.1016/j.ajog.2010.04.006.
Wu J, Zhang J, Yang J, Zheng TQ, Chen Y-M. Association between platelet indices and risk of preeclampsia in pregnant women. J Obstet Gynaecol. 2022;42:2764–70. https://doi.org/10.1080/01443615.2022.2109136.
Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021;10. https://doi.org/10.3390/cells10113055.
Gogoi P, Sinha P, Gupta B, Firmal P, Rajaram S. Neutrophil-to-lymphocyte ratio and platelet indices in pre-eclampsia. Int J Gynaecol Obstet. 2019;144:16–20. https://doi.org/10.1002/ijgo.12701.
Serin S, Avcı F, Ercan O, Köstü B, Bakacak M, Kıran H. Is neutrophil/lymphocyte ratio a useful marker to predict the severity of pre-eclampsia? Pregnancy Hypertens. 2016;6:22–5. https://doi.org/10.1016/j.preghy.2016.01.005.
Lin S, Zhang L, Shen S, Wei D, Lu J, Chen X, et al. Platelet parameters and risk of hypertension disorders of pregnancy: a propensity score adjusted analysis. Platelets. 2022;33:543–50. https://doi.org/10.1080/09537104.2021.1945569.
Moser G, Guettler J, Forstner D, Gauster M. Maternal platelets—friend or foe of the human placenta? Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20225639.
Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood. 2016;128:2153–64.
Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. https://doi.org/10.1007/s00018-009-0205-1.
Giaglis S, Stoikou M, Grimolizzi F, Subramanian BY, van Breda SV, Hoesli I, et al. Neutrophil migration into the placenta: good, bad or deadly? Cell Adh Migr. 2016;10:208–25. https://doi.org/10.1080/19336918.2016.1148866.
Walsh SW. Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 2007;18:365–70.
Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol. 2014;5:298 https://doi.org/10.3389/fimmu.2014.00298.
Vishnyakova P, Elchaninov A, Fatkhudinov T, Sukhikh G. Role of the monocyte-macrophage system in normal pregnancy and preeclampsia. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20153695.
Ning F, Liu H, Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol. 2016;75:298–309. https://doi.org/10.1111/aji.12477.
Major HD, Campbell RA, Silver RM, Branch DW, Weyrich AS. Synthesis of sFlt-1 by platelet-monocyte aggregates contributes to the pathogenesis of preeclampsia. Am J Obstet Gynecol. 2014;210:547.e1–e7. https://doi.org/10.1016/j.ajog.2014.01.024.
Zhao Q, Liu R, Chen H, Yang X, Dong J, Bai M, et al. Higher circulating lymphocytes and the incidence of pre-eclampsia and eclampsia. J Pregnancy. 2024;2024:8834312 https://doi.org/10.1155/2024/8834312.
Bueno-Sánchez JC, Agudelo-Jaramillo B, Escobar-Aguilerae LF, Lopera A, Cadavid-Jaramillo AP, Chaouat G, et al. Cytokine production by non-stimulated peripheral blood NK cells and lymphocytes in early-onset severe pre-eclampsia without HELLP. J Reprod Immunol. 2013;97:223–31. https://doi.org/10.1016/j.jri.2012.11.007.
Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A, et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol. 2019;234:5106–16. https://doi.org/10.1002/jcp.27315.
Tangerås LH, Austdal M, Skråstad RB, Salvesen KÅ, Austgulen R, Bathen TF, et al. Distinct first trimester cytokine profiles for gestational hypertension and preeclampsia. Arterioscler Thromb Vasc Biol. 2015;35:2478–85. https://doi.org/10.1161/ATVBAHA.115.305817.
Hu B, Yang X-R, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. https://doi.org/10.1158/1078-0432.CCR-14-0442.
Zinellu A, Collu C, Nasser M, Paliogiannis P, Mellino S, Zinellu E, et al. The aggregate index of systemic inflammation (AISI): a novel prognostic biomarker in idiopathic pulmonary fibrosis. J Clin Med. 2021;10. https://doi.org/10.3390/jcm10184134.
Zhang YR, Wang JJ, Chen SF, Wang HF, Li YZ, Ou YN, et al. Peripheral immunity is associated with the risk of incident dementia. Mol Psychiatry. 2022;27:1956–62. https://doi.org/10.1038/s41380-022-01446-5.
Xiu J, Lin X, Chen Q, Yu P, Lu J, Yang Y, et al. The aggregate index of systemic inflammation (AISI): a novel predictor for hypertension. Front Cardiovasc Med. 2023;10:1163900 https://doi.org/10.3389/fcvm.2023.1163900.
Xie S, Zhang E, Gao S, Su S, Liu J, Zhang Y, et al. Associations of systemic immune-inflammation index and systemic inflammation response index with maternal gestational diabetes mellitus: evidence from a prospective birth cohort study. Chin Med J. 2025;138:729–37. https://doi.org/10.1097/cm9.0000000000003236.
Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841–8. https://doi.org/10.1007/s10654-021-00752-6.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381 https://doi.org/10.1136/bmj.l2381.
Gunderson EP, Greenberg M, Nguyen-Huynh MN, Tierney C, Roberts JM, Go AS, et al. Early pregnancy blood pressure patterns identify risk of hypertensive disorders of pregnancy among racial and ethnic groups. Hypertension. 2022;79:599–613. https://doi.org/10.1161/HYPERTENSIONAHA.121.18568.
Leonard SA, Formanowski BL, Phibbs CS, Lorch S, Main EK, Kozhimannil KB, et al. Chronic hypertension in pregnancy and racial–ethnic disparities in complications. Obstet Gynecol. 2023;142:862–71. https://doi.org/10.1097/aog.0000000000005342.
Acknowledgements
We extend our gratitude to all the pregnant women who participated in this study. Additionally, we sincerely thank the clinicians at Qingdao Women and Children’s Hospital, affiliated with Qingdao University, for their invaluable contributions in collecting samples and performing laboratory tests.
Funding
This work was supported by a grant from the Municipal Key Clinical Specialty Programs of Qingdao.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y-LW and X-JK; Data curation: Z-XG and J-HD; Formal analysis: BH and Y-TJ; Project administration: X-JK; Supervision: J-YW; Validation: SK; Roles/Writing—original draft: X-JK and SK; Writing—review & editing: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, X., Guo, Z., Dong, J. et al. Association of first trimester peripheral blood count-derived immune markers with the risk of incident hypertensive disorders of pregnancy: a retrospective cohort study. Hypertens Res (2026). https://doi.org/10.1038/s41440-026-02571-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41440-026-02571-2