Abstract
Glucagon-like peptide-1 (GLP-1) receptor agonists, widely prescribed for type 2 diabetes and weight management, are known for their metabolic benefits but may have unrecognized side effects. This study investigates the association between GLP-1 receptor agonists and male sexual dysfunction using data from the FDA Adverse Event Reporting System (FAERS). Reports from Q4 2003 to Q1 2024 were analyzed using the OpenVigil 2.1 platform to identify male patients experiencing orgasmic dysfunction, erectile dysfunction, or decreased libido linked to GLP-1 receptor agonists (tirzepatide, semaglutide, dulaglutide, exenatide, lixisenatide, and liraglutide). After cleaning duplicate entries, disproportionality measures (reporting odds ratio (ROR), proportional reporting ratio (PRR), and relative reporting ratio (RRR)) were calculated, with Evans’ criteria applied to assess signal significance. Among 182 cases identified, patients were predominantly aged 40–60 years, with exenatide accounting for 24.2% of reports, followed by semaglutide (21.4%). Diabetes was the most common indication (43.9%). Despite statistically significant chi-squared values (P < 0.0001), low ROR (0.41, 95% Confidence interval (CI): 0.36–0.48), PRR (0.41, 95% CI: 0.36–0.48), and RRR (0.42, 95% CI: 0.36–0.48) suggest a weak association. These findings underscore the need for monitoring as GLP-1 use expands, though overall patient risk remains low.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications commonly prescribed for the management of type 2 diabetes mellitus (T2DM), heart failure and weight loss [1]. These agents mimic the incretin hormone GLP-1 by enhancing glucose-dependent insulin secretion, inhibiting glucagon release, and slowing gastric emptying. These collectively contribute to improved glycemic control and weight loss. Common GLP-1 receptor agonists include semaglutide, exenatide, liraglutide, and dulaglutide [2].
The use of GLP-1 receptor agonists has become increasingly prevalent. In the United States, 6% of adults are currently using a GLP-1 agonist and about one in eight adults (12%) have ever taken such a drug [3]. Among adults diagnosed with diabetes, the usage rate rises significantly, with 43% reporting they have used a GLP-1 agonist [3]. Among adolescents, prescriptions for GLP-1 agonists have increased nearly 600% over the past four years suggesting the medication is widely used across all ages in the US [4].
Gastrointestinal issues such as nausea, vomiting, diarrhea, and abdominal pain are among the most frequently reported adverse reactions [5]. While GLP-1 receptor agonists are generally well-tolerated [6], there is evidence from animal studies that these medications may impact sexual function [7, 8]. Specifically, studies have shown that GLP-1 receptors in the brain, particularly within areas associated with reward processing such as the laterodorsal tegmental area, ventral tegmental area, and nucleus accumbens shell, may play a role in modulating sexual behaviors. Activation of GLP-1 receptors in these areas has been shown to reduce sexual interaction behaviors in male mice, suggesting that GLP-1 receptor agonists could impact orgasmic function by influencing the neurocircuitry involved in sexual reward and motivation [7, 8].
Orgasm involves increased brain activity in specific regions, such as the occipitotemporal, anterior cingulate, and insular cortices, and decreased activity in the prefrontal cortex, as demonstrated by advances in functional neuroimaging [9]. Orgasm dysfunction is defined as the persistent or recurrent difficulty, delay in, or absence of attaining orgasm after sufficient sexual stimulation, which can stem from various etiologies such as medications, neurogenic causes, endocrinopathies, and psychological factors [10]. Similarly, erectile dysfunction (ED) can result from any disease process affecting penile arteries, nerves, hormone levels, smooth muscle tissue, and corporal endothelium [11, 12]. The prevalence of decreased libido, orgasmic dysfunction, and ED were reported as 2.9, 8, and 24.2% in the US population, respectively [13,14,15].
In this study, we extracted and analyzed data from the FDA Adverse Event Reporting System (FAERS) database on cases of various male sexual dysfunctions associated with GLP-1 receptor agonists. Our aim was to better understand the patient characteristics and outcomes of these adverse events. By shedding light on this underreported side effect, we hope to inform clinical practice and guide future research on the safe use of GLP-1 receptor agonists in the management of T2DM.
Method
Data source
This study utilized data from FAERS, a surveillance database that collects information on adverse drug reactions. The FAERS database is updated quarterly and follows the International Conference on Harmonization’s (ICH) international safety reporting guidelines. The data extraction covered reports from the fourth quarter of 2003 to the first quarter of 2024.
Study design
A retrospective disproportionality analysis was performed to identify reports of male sexual dysfunction associated with GLP-1 receptor agonists. The analysis employed the OpenVigil 2.1 platform, a tool designed to provide clean and curated access to FAERS data. This platform was used to extract, filter, and analyze the data using disproportionality measures such as the reporting odds ratio (ROR), proportional reporting ratio (PRR), and relative reporting ratio (RRR).
Search strategy and data extraction
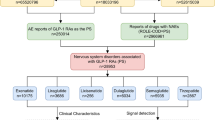
An advanced search and analysis were conducted on the OpenVigil 2.1 platform to identify cases of male sexual dysfunction associated with specific GLP-1 receptor agonists by including multiple relevant MedDRA preferred terms (PT). The drugs included were tirzepatide, semaglutide, dulaglutide, exenatide, lixisenatide, and liraglutide. The adverse events considered were ‘anorgasmia’, ‘orgasm abnormal’, ‘libido decreased’, ‘libido disorder’, ‘loss of libido’, ‘erectile dysfunction’, ‘organic erectile dysfunction’, ‘psychogenic erectile dysfunction’, ‘male sexual dysfunction’, ‘sexual dysfunction’, ‘genital hypoaesthesia’, ‘hypoaesthesia of genital male’, ‘ejaculation disorder’, ‘ejaculation failure’, ‘premature ejaculation’, ‘painful ejaculation’, and ‘painful erection’.
Following best practices for data cleaning in pharmacovigilance studies, we removed duplicate cases with same case id and selected cases where the drug was identified as the primary suspect. Only male cases were included in this study. Related subgroups were consolidated into broader categories, such as grouping all orgasm-related issues under ‘Orgasm abnormal,’ and etc.
Statistical analysis
Disproportionality measures were calculated to assess the strength of the association between GLP-1 receptor agonists and orgasm dysfunction. The following measures were used (Table 1):
Reporting odds ratio (ROR)
Calculated as \(\frac{(A/B)}{(C/D)}\), where A is the number of reports containing both the suspected drug and the suspected adverse drug reaction (ADR), B is the number of reports containing the drug of choice but with another ADR, C is the number of reports containing the event of interest but with other medications, and D is the number of reports containing other medications and other ADRs. The standard error (SE) was calculated as \(\sqrt{\frac{1}{A}+\frac{1}{B}+\frac{1}{C}+\frac{1}{D}}\). A signal was considered significant if ROR - 1.96SE > 1.
Proportional reporting ratio (PRR)
Calculated as \(\frac{A/(A+C)}{B/(B+D)}\), with SE calculated as \(\sqrt{\frac{1}{A}-\frac{1}{A+C}+\frac{1}{B}-\frac{1}{B+D}}\). A signal was considered significant if PRR ≥ 2, with a chi-squared value > 4, and at least three reports of the preferred term (PT).
Relative reporting ratio (RRR)
Similar to ROR, calculated as \(\frac{(A/B)}{(C/D)}\), it provides a relative measure of the reporting rate of the adverse event for the drug of interest compared to all other drugs.
The thresholds for detecting a signal were defined by Evans’ criteria as follows [16]:
-
ROR: ROR - 1.96SE > 1
-
PRR: PRR ≥ 2, with a chi-squared value > 4, and at least three reports of the PT
These measures were used to determine the statistical significance of the association between GLP-1 receptor agonists and orgasm dysfunction. An association was considered statistically significant if the lower limit of the 95% confidence interval (CI) was ≥1.0. Statistical significance was determined at P < 0.05.
The results were interpreted by comparing the observed frequencies of orgasm dysfunction associated with GLP-1 receptor agonists against expected frequencies based on the entire FAERS dataset. Disproportionality analysis was used to identify whether the observed associations were statistically significant and whether they could indicate a potential causal relationship.
Results
The analysis identified a total of 182 cases of adverse sexual events associated with GLP-1 receptor agonists.
Age distribution
The age distribution of reported cases in FAERS is depicted in Table 2. The majority of reports were from individuals with a median patient age of 55 years (IQR: 48–62 years).
Annual distribution of reports
The annual distribution of adverse event reports related to male sexual dysfunction from 2005–2024 is shown in Fig. 1. There is an apparent increase in the number of reports in recent years, particularly in 2023. The number of reports for semaglutide has shown an increasing trend in recent years, while tirzepatide surpassed semaglutide in 2023 and the first quarter of 2024.
The thin light gray line represents the total number of reports per year. Individual drugs are distinguished by bar styles: dulaglutide (black horizontal stripes), exenatide (diagonal stripes), liraglutide (light gray with horizontal stripes), lixisenatide (solid gray), semaglutide (light gray with black border), and tirzepatide (dark gray with black border).
FAERS data analysis
Table 2 summarizes the demographic characteristics, levels of the suspect drug, and routes of administration for these cases. The majority of reports originated from the United States, followed by European countries, China, Mexico, Canada, Israel, Japan, and Lebanon. Of 182 reports, most indicated that the GLP-1 receptor agonists were the primary suspect, with subcutaneous administration being the most common route (N = 102, 56.0%).
Distribution of reports by GLP-1 receptor agonist
The distribution of reports for different GLP-1 receptor agonists is summarized in Table 2. Exenatide had the highest number of reports (N = 44, 24.2%), followed by semaglutide (N = 39, 21.4%) and liraglutide (N = 38, 20.9%).
Indications and adverse events
The distribution of indications for the use of GLP-1 receptor agonists and the associated adverse events are presented in Table 3. Diabetes mellitus was the most common indication (N = 80, 43.9%), followed by weight loss (N = 9, 4.9%). 51.2% of cases did not specify an indication.
Disproportionality analysis
Table 4 summarizes the ROR, RRR, PRR, and chi-squared values for each GLP-1 receptor agonist. The chi-squared values for semaglutide, dulaglutide, exenatide, liraglutide, and the total all exceed 4, indicating significant differences in the observed versus expected frequencies of general adverse events (P < 0.0001). However, tirzepatide and lixisenatide did not exceed this threshold. Despite the chi-squared values indicating statistical significance for several drugs, the low values of ROR (0.41, 95% CI: 0.36–0.48), RRR (0.42, 95% CI: 0.36–0.48), and PRR (0.41, 95% CI: 0.36–0.48) suggest that the observed association between GLP-1 receptor agonists and male sexual dysfunction is not strong enough to warrant significant clinical concern. Evans’ criteria determined there is likely no relationship between male sexual dysfunction and administration of GLP-1 agonists with the current data. Moreover, the clinical relevance of these findings is further diminished by the wide confidence intervals, which indicate considerable uncertainty around the estimates.
Subcategorical analysis of male sexual dysfunction
To further investigate the potential associations between GLP-1 receptor agonists and male sexual dysfunction, we analyzed specific subcategories, including ED and orgasm dysfunction. The chi-squared values for total GLP-1 receptor agonists are greater than 4 (ED: 142.74 and orgasm dysfunction: 71.69), indicating significant difference in the observed frequencies of these events compared to the general adverse events (P < 0.0001).For ED, the disproportionality measures were as follows: RRR 0.50 (95% CI, 0.42–0.59), PRR 0.50 (95% CI, 0.42–0.58), and ROR 0.50 (95% CI, 0.42–0.58). For orgasm dysfunction, the measures were RRR 0.42 (95% CI, 0.36–0.48), PRR 0.41 (95% CI, 0.36–0.48), and ROR 0.41 (95% CI, 0.36–0.48).
Discussion
In our study, we identified numerous reports of male sexual dysfunction in the FAERS database; however, further analysis suggests that the observed association is weak and may lack clinical significance. Although chi-squared values indicated statistically significant differences in reporting frequencies (P < 0.0001), they do not quantify the strength of the association between GLP-1 receptor agonists and male sexual dysfunction. While disproportionality measures such as ROR, PRR, and RRR are standard in pharmacovigilance for signal detection, their nearly identical values in this dataset (ROR: 0.41, PRR: 0.41, RRR: 0.42) suggest a weak association with limited clinical concern. However, using multiple measures enhances consistency and robustness, as different metrics may perform variably across datasets [17, 18]. Additionally, the wide confidence intervals reflect uncertainty in the signal estimates [19], which may be attributed to the relatively small number of reports in certain subcategories.
The results of our study highlight the complexities in assessing the safety profile of GLP-1 receptor agonists regarding sexual dysfunction in male patients. There was a noticeable increase in the number of adverse event reports in recent years, particularly in 2023. In this year, the clinical use of GLP-1 receptor agonists expanded significantly, primarily driven by their increasing use for weight loss, which was recognized as the breakthrough of the year by Science journal [20, 21]. The spike in reports during this year could be attributed to this broader clinical application, as well as heightened awareness among healthcare providers and patients regarding manageable side effects [21]. Moreover, considering we are only in the middle of 2024, it is plausible that the number of reported cases could surpass those of 2023, further supporting the need for ongoing surveillance. Our findings indicate that exenatide had the highest number of reports, followed by semaglutide. This trend may reflect differences in the FDA approval dates and the duration of availability of these GLP-1 receptor agonists. Exenatide, approved by FDA in April 2005, has been on the market significantly longer than semaglutide, which received FDA approval in December 2017 [22, 23]. However, it is noteworthy that semaglutide has gained considerable prominence in recent years, potentially surpassing other GLP-1 receptor agonists in terms of usage in weight management and glycemic control [24]. Interestingly, our findings indicate that tirzepatide has exhibited a higher number of adverse event reports related to male sexual dysfunction in recent years. This trend may be attributed to tirzepatide’s relatively recent FDA approval in May 2022 and its significantly higher growth rate in usage (215%) compared to semaglutide [24, 25]. This rapid adoption could be linked to tirzepatide’s superior efficacy in promoting weight loss compared to semaglutide, making it a preferred choice among patients and healthcare providers [26]. These trends emphasize the importance of continuous monitoring as these drugs become even more commonplace in clinical practice.
When comparing these findings with other studies on GLP-1 receptor agonists, some contrasting results emerge. Able et al. also found that non-diabetic, obese men prescribed semaglutide were significantly more likely to develop ED compared to those who did not receive the prescription (1.47% vs 0.32%) [27]. This further emphasizes the potential sexual side effects of GLP-1 receptor agonists and the need for careful monitoring of patients undergoing such treatments. Conversely, other studies suggest a protective or therapeutic effect of GLP-1 receptor agonists on ED, particularly in diabetic patients. Liraglutide, for example, demonstrated improvements in erectile function when combined with lifestyle modifications, metformin, and testosterone therapy in diabetic obese men with hypogonadism [28]. Similarly, dulaglutide was found to ameliorate ED, suggesting potential benefits for addressing sexual dysfunction [29]. These studies collectively highlight the multifaceted effects of GLP-1 receptor agonists on sexual function, which appear to vary based on patient comorbidities and concurrent treatments.
Additionally, recent research has highlighted other adverse effects associated with GLP-1 receptor agonists. For instance, a study by Hathaway et al. reported an association between semaglutide and an increased risk of nonarteritic anterior ischemic optic neuropathy (NAION) [30]. This finding broadens the spectrum of potential adverse effects of GLP-1 receptor agonists to include vascular conditions and underscores the importance of ongoing surveillance and research into their safety profiles. Interestingly, some studies, such as Arillotta et al. [31], report an increase in libido among users of GLP-1 receptor agonists, which contrasts with our findings that highlight a potential association with decreased libido. This suggests that the effects of GLP-1 receptor agonists on sexual function may be multifaceted and warrant further investigation. Indeed, weight loss, a known outcome of GLP-1 receptor use, may improve sexual desire in men.
The mechanisms by which GLP-1 receptor agonists might influence sexual function are not fully understood. GLP-1 receptors are expressed in various brain regions involved in the regulation of appetite and reward, such as the hypothalamus and brainstem [32]. Activation of these receptors can influence the release of neurotransmitters like dopamine and serotonin, which are also involved in sexual arousal and orgasm [33, 34]. Animal studies have shown that GLP-1 can cross the blood-brain barrier and modulate neural activity, potentially affecting pathways involved in sexual desire and orgasm [7, 8, 32]. However, the translation of these findings from animal models to human patients requires further investigation.
Given our results, clinicians should remain vigilant but not overly concerned about the potential for male sexual dysfunction when prescribing GLP-1 receptor agonists. It is important to consider these findings in the context of the overall benefit-risk profile of GLP-1 receptor agonists, which are highly effective in managing blood glucose levels and promoting weight loss in patients with T2DM.
Strengths and limitations
This study is the first to report the potential association of male sexual dysfunction with all GLP-1 agonists in a real-world setting based on provider and consumer reports. A key strength is the use of the FAERS database, which provides a large and comprehensive dataset for detecting rare adverse events. Advanced search techniques and disproportionality analysis on the OpenVigil 2.1 platform allowed for a thorough examination of potential associations between GLP-1 receptor agonists and male sexual dysfunction. While previous studies found improvements in sexual dysfunction with GLP-1 agonists and, to the best of our knowledge, only one evaluated the possibility of ED with semaglutide, our analysis covers all GLP-1 agonists and various forms of male sexual dysfunction, providing broader insights.
However, several limitations should be acknowledged. First, the spontaneous nature of the FAERS database inherently carries limitations such as underreporting, reporting bias, and lack of comprehensive clinical context, including patient comorbidities, medication adherence, and duration of treatment. Disproportionality measures alone cannot establish causation or provide an absolute quantification of risk but serve as a preliminary indicator requiring further clinical validation through controlled studies. Another limitation is that our analysis can only suggest a possible link between GLP-1 receptor agonists and male sexual dysfunction; it cannot prove causation. This is because disproportionality analysis identifies associations but does not control for other variables that could affect the outcome. Additionally, the presence of comorbidities that already put patients at high risk for ED and male sexual dysfunction could confound the results. The study applied commonly used disproportionality measures such as ROR, PRR, and chi-squared values for signal detection in pharmacovigilance studies [16, 35]. These methodologies are well-established but did not incorporate adjustments for multiple comparisons in separate analyses of erectile dysfunction and orgasm dysfunction. Future research could adopt statistical corrections to mitigate this limitation. While disproportionality analysis inherently accounts for differences in reporting rates across drugs and events, it does not fully correct for multiple hypothesis testing. Given our exploratory approach with separate analyses for ED and orgasm dysfunction subcategories, future confirmatory studies should incorporate statistical corrections (e.g., Bonferroni adjustment [36]) to mitigate the risk of false positives.
Although the FAERS database reports brand names of drugs, it does not explicitly differentiate between brand-name medications and compounded versions, which may introduce variability in the reported outcomes. This heterogeneity in preparation could affect the consistency of the adverse event data and warrants caution when interpreting the findings. Furthermore, the study lacks detailed clinical information on the patients, such as the duration of drug use, baseline health status, and lifestyle factors that could confound the results. Despite these limitations, this study provides valuable insights into the potential side effects of GLP-1 receptor agonists and highlights the need for further research in this area.
Conclusions
While our analysis detected significant statistical signals in reporting frequencies, the low disproportionality measures and wide confidence intervals suggest that the association between GLP-1 receptor agonists and male sexual dysfunction is weak. These findings emphasize the need for cautious interpretation of pharmacovigilance data and further research to confirm or refute potential associations.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Mashayekhi M, Beckman JA, Nian H, Garner EM, Mayfield D, Devin JK, et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: a randomized controlled trial. Diabetes Obes Metab. 2023;25:570–80. https://doi.org/10.1111/dom.14903
Ayoub M, Aibani R, Dodd T, Ceesay M, Bhinder M, Faris C, et al. Risk of esophageal and gastric cancer in patients with type 2 diabetes receiving glucagon-like peptide-1 receptor agonists (GLP-1 RAs): A national analysis. Cancers. 2024;16:3224. https://doi.org/10.3390/cancers16183224
Montero A, Sparks G, Presiado M, Hamel L.: KFF health tracking poll may 2024: the public’s use and views of GLP-1 drugs. https://www.kff.org/health-costs/poll-finding/kff-health-tracking-poll-may-2024-the-publics-use-and-views-of-glp-1-drugs/ (2024). Accessed May 24 2024.
Lee JM, Sharifi M, Oshman L, Griauzde DH, Chua K-P. Dispensing of glucagon-like peptide-1 receptor agonists to adolescents and young adults, 2020–2023. JAMA. 2024;331:2041–3. https://doi.org/10.1001/jama.2024.7112
Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: a real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol. 2022;13:1043789. https://doi.org/10.3389/fendo.2022.1043789
Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38:390–402. https://doi.org/10.2337/cd19-0100
Vestlund J, Jerlhag E. The glucagon-like peptide-1 receptor agonist, exendin-4, reduces sexual interaction behaviors in a brain site-specific manner in sexually naive male mice. Horm Behav. 2020;124:104778 https://doi.org/10.1016/j.yhbeh.2020.104778
Vestlund J, Jerlhag E. Glucagon-like peptide-1 receptors and sexual behaviors in male mice. Psychoneuroendocrinology. 2020;117:104687. https://doi.org/10.1016/j.psyneuen.2020.104687
Georgiadis JR, Reinders AA, Van der Graaf FH, Paans AM, Kortekaas R. Brain activation during human male ejaculation revisited. Neuroreport. 2007;18:553–7. https://doi.org/10.1097/WNR.0b013e3280b10bfe
Rowland D, McMahon CG, Abdo C, Chen J, Jannini E, Waldinger MD, et al. Disorders of orgasm and ejaculation in men. J Sex Med. 2010;7:1668–86. https://doi.org/10.1111/j.1743-6109.2010.01782.x
Jie HW, Jie W, Jianxiong M, Xin Z, Runnan X, Yijia F, et al. Mechanism of denervation muscle atrophy mediated by Ach/p38/MAPK pathway in rats with erectile dysfunction caused by nerve injury. Exp Cell Res. 2024;442:114283. https://doi.org/10.1016/j.yexcr.2024.114283
Jin X, Sun L, Li H, Liu Y. Association between the composite dietary antioxidant index and erectile dysfunction in US men: a cross-sectional study. J Health Popul Nutr. 2024;43:184. https://doi.org/10.1186/s41043-024-00653-w
Quinta Gomes AL, Nobre PJ. Prevalence of sexual problems in Portugal: results of a population-based study using a stratified sample of men aged 18 to 70 years. J Sex Res. 2014;51:13–21. https://doi.org/10.1080/00224499.2012.744953
Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–44. https://doi.org/10.1001/jama.281.6.537
Mark KP, Arenella K, Girard A, Herbenick D, Fu J, Coleman E. Erectile dysfunction prevalence in the United States: report from the 2021 national survey of sexual wellbeing. J Sex Med. 2024;21:296–303. https://doi.org/10.1093/jsxmed/qdae008
Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6. https://doi.org/10.1002/pds.677
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23. https://doi.org/10.1002/pds.1001
Böhm R.: Primer on disproportionality analysis. https://openvigil.sourceforge.net/doc/DPA.pdf (2018). Accessed 03/17/2025.
Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ. 1995;311:485. https://doi.org/10.1136/bmj.311.7003.485
Watanabe JH, Kwon J, Nan B, Reikes A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J Am Pharm Assoc. 2024;64:133–8. https://doi.org/10.1016/j.japh.2023.10.002
Thorp HH. More questions than answers. Science. 2023;382:1213 https://doi.org/10.1126/science.adn3693
(FDA) USFaDA: FDA approves new drug treatment for chronic weight management, first since 2014. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014 (2021). Accessed Nov 2 2024.
(FDA) USFaDA: Drug approval package byetta (Exenatide) injection. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021773_byettatoc.cfm#:~:text=Approval%20Date%3A%204%2F28%2F2005 (2005). Accessed Nov 2 2024.
Jagielski D.: These were the top-selling GLP-1 drugs last quarter from Eli Lilly and Novo Nordisk. https://finance.yahoo.com/news/were-top-selling-glp-1-120000543.html (2024). Accessed Nov 2, 2024.
(FDA) USFaDA: Drug trials snapshots: MOUNJARO. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-mounjaro (2022). Accessed Nov 2 2024.
Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, Brar R, Baker C, Gluckman TJ, et al. Semaglutide vs Tirzepatide for weight loss in adults with overweight or obesity. JAMA Intern Med. 2024;184:1056–64. https://doi.org/10.1001/jamainternmed.2024.2525
Able C, Liao B, Saffati G, Maremanda A, Applewhite J, Nasrallah AA, et al. Prescribing semaglutide for weight loss in non-diabetic, obese patients is associated with an increased risk of erectile dysfunction: A TriNetX database study. Int J Impot Res. 2024. https://doi.org/10.1038/s41443-024-00895-6
Giagulli VA, Carbone MD, Ramunni MI, Licchelli B, De Pergola G, Sabba C, et al. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology. 2015;3:1094–103. https://doi.org/10.1111/andr.12099
Bajaj HS, Gerstein HC, Rao-Melacini P, Basile J, Colhoun H, Conget I, et al. Erectile function in men with type 2 diabetes treated with dulaglutide: an exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinol. 2021;9:484–90. https://doi.org/10.1016/S2213-8587(21)00115-7
Hathaway JT, Shah MP, Hathaway DB, Zekavat SM, Krasniqi D, Gittinger JW Jr., et al. Risk of nonarteritic anterior ischemic optic neuropathy in patients prescribed semaglutide. JAMA Ophthalmol. 2024;142:732–9. https://doi.org/10.1001/jamaophthalmol.2024.2296
Arillotta D, Floresta G, Papanti Pelletier GD, Guirguis A, Corkery JM, Martinotti G, et al. Exploring the potential impact of GLP-1 receptor agonists on substance use, compulsive behavior, and libido: insights from social media using a mixed-methods approach. Brain Sci. 2024;14:617 https://doi.org/10.3390/brainsci14060617
Fu Z, Gong L, Liu J, Wu J, Barrett EJ, Aylor KW, et al. Brain endothelial cells regulate glucagon-like peptide 1 entry into the brain via a receptor-mediated process. Front Physiol. 2020;11:555. https://doi.org/10.3389/fphys.2020.00555
Lengsfeld S, Hasenbohler F, Probst L, Baur F, Emara Y, Bathelt C, et al. Effects of glucagon-like peptide-1 analogs on sexual desire: a randomized, double-blind, placebo-controlled crossover trial. Endocr Abstr. 2023. https://doi.org/10.1530/endoabs.90.P69.
Brunetti L, Orlando G, Recinella L, Leone S, Ferrante C, Chiavaroli A, et al. Glucagon-like peptide 1 (7–36) amide (GLP-1) and exendin-4 stimulate serotonin release in rat hypothalamus. Peptides. 2008;29:1377–81. https://doi.org/10.1016/j.peptides.2008.04.007
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36. https://doi.org/10.1002/pds.1742
Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34:502–8. https://doi.org/10.1111/opo.12131
Author information
Authors and Affiliations
Contributions
APL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing; ALC: Conceptualization, Writing, review & editing. SB: Methodology, Visualization, Writing, review & editing; ERS: Writing draft, review & editing, JS: Writing draft, review & editing; YSC: Supervision, Writing, review & editing, FDG: Writing draft, review & editing; MS: review & editing; MLE: Conceptualization, Methodology, Supervision, Validation, Visualization, review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with relevant guidelines and regulations. This study utilized publicly available, de-identified data from the FDA Adverse Event Reporting System (FAERS), and therefore did not involve direct interaction with human participants or identifiable private information. As such, the Stanford University School of Medicine Institutional Review Board determined that the study was exempt from review (IRB exemption granted; no reference number applicable for de-identified dataset studies). Because the research did not involve the collection of identifiable human data or images, informed consent to participate and consent for publication were not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourabhari Langroudi, A., Chen, A.L., Basran, S. et al. Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data. Int J Impot Res 37, 661–667 (2025). https://doi.org/10.1038/s41443-025-01061-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41443-025-01061-2
This article is cited by
-
Comment on: Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data
International Journal of Impotence Research (2025)
-
Comment on: Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data
International Journal of Impotence Research (2025)
-
Response to Comment on: Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data
International Journal of Impotence Research (2025)
-
Response to Comment on: Male sexual dysfunction associated with GLP-1 receptor agonists: a cross-sectional analysis of FAERS data
International Journal of Impotence Research (2025)