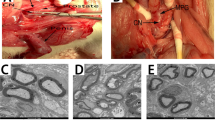
Abstract
Erectile dysfunction following non-nerve-sparing prostatectomy is prevalent. Although adipose-derived stem cells (ASCs) have shown therapeutic potential for erectile dysfunction, the underlying mechanisms remain unclear. To investigate the therapeutic effects of exogenous stem cells and their recruitment of endogenous stem cells in the context of postprostatectomy erectile dysfunction, we established a bilateral cavernous nerve injury (BCNI) rat model. The rats were divided into the sham surgery, BCNI + PBS, and BCNI+ASCs groups. Intracavernous pressure/mean arterial pressure ratios were compared between groups. In vivo imaging revealed ASC retention in the corpora cavernosa. Penile histomorphology and smooth muscle content were evaluated via Hematoxylin‒eosin and Masson staining. Immunohistochemistry, western blotting, and immunofluorescence were employed to quantify α-SMA, nNOS, caspase-3, vWF, Bax, Bcl-2, and SDF-1 levels and endogenous stem cell recruitment. Compared with the PBS control group, the ASC-treated group presented increased α-SMA, nNOS, and vWF expression and smooth muscle content, as well as reduced caspase-3, Bax, and Bcl-2 levels. Endogenous stem cell recruitment was observed in ASC-treated rats. Our findings demonstrate that exogenous ASCs injection suppresses apoptosis, enhances endothelial function, recruits endogenous stem cells, and alleviates erectile dysfunction in BCNI models. This study elucidates ASC-mediated mechanisms involved in erectile dysfunction recovery, providing insights for clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492
Hugosson J, Godtman RA, Wallstrom J, Axcrona U, Bergh A, Egevad L, et al. Results after four years of screening for prostate cancer with PSA and MRI. N Engl J Med. 2024;391:1083–95. https://doi.org/10.1056/NEJMoa2406050
Hugosson J, Månsson M, Wallström J, Axcrona U, Carlsson SV, Egevad L, et al. Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N Engl J Med. 2022;387:2126–37. https://doi.org/10.1056/NEJMoa2209454
Winter A, Sirri E, Jansen L, Wawroschek F, Kieschke J, Castro FA, et al. Comparison of prostate cancer survival in Germany and the USA: can differences be attributed to differences in stage distributions? BJU Int. 2017;119:550–9. https://doi.org/10.1111/bju.13537
Reddy D, Peters M, Shah TT, van Son M, Tanaka MB, Huber PM, et al. Cancer Control outcomes following focal therapy using high-intensity focused ultrasound in 1379 men with nonmetastatic prostate cancer: a multi-institute 15-year experience. Eur Urol. 2022;81:407–13. https://doi.org/10.1016/j.eururo.2022.01.005
Aker MN, Brisbane WG, Kwan L, Gonzalez S, Priester AM, Kinnaird A, et al. Cryotherapy for partial gland ablation of prostate cancer: oncologic and safety outcomes. Cancer Med. 2023;12:9351–62. https://doi.org/10.1002/cam4.5692
Derks YHW, Schilham MGM, Rijpkema M, Smeets EMM, Amatdjais-Groenen HIV, Kip A, et al. Imaging and photodynamic therapy of prostate cancer using a theranostic PSMA-targeting ligand. Eur J Nucl Med Mol Imaging. 2023;50:2872–84. https://doi.org/10.1007/s00259-023-06224-1
Chao B, Lepor H. 5-Year outcomes following focal laser ablation of prostate cancer. Urology. 2021;155:124–9. https://doi.org/10.1016/j.urology.2021.03.054
Pikramenos K, Zachou M, Papadopoulos D, Papatsoris A, Varkarakis I, Mitsogiannis I. Post radical prostatectomy erectile dysfunction. a single centre experience. Cureus. 2023;15:e34601. https://doi.org/10.7759/cureus.34601
Leslie SW, Sooriyamoorthy T Erectile dysfunction. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
Vance G, Zeigler-Hill V, James RM, Shackelford TK. Erectile dysfunction and partner-directed behaviors in romantic relationships: the mediating role of suspicious jealousy. J Sex Res. 2022;59:472–83. https://doi.org/10.1080/00224499.2021.1956412
Vance G, Zeigler-Hill V, Meehan MM, Young G, Shackelford TK. Erectile dysfunction, suspicious jealousy, and partner-directed behaviors in heterosexual romantic couples. Arch Sex Behav. 2023;52:3139–53. https://doi.org/10.1007/s10508-023-02672-w
Pellegrino F, Sjoberg DD, Tin AL, Benfante NE, Briganti A, Montorsi F, et al. Relationship between age, comorbidity, and the prevalence of erectile dysfunction. Eur Urol Focus. 2023;9:162–7. https://doi.org/10.1016/j.euf.2022.08.006
Xiong Y, Zhang F, Zhang Y, Wang W, Ran Y, Wu C, et al. Insights into modifiable risk factors of erectile dysfunction, a wide-angled Mendelian Randomization study. J Adv Res. 2024;58:149–61. https://doi.org/10.1016/j.jare.2023.05.008
Qalawena MM, Al-Shatouri MA, Motawaa MA, El-Sakka AI. Association between prostate zonal volume and erectile dysfunction in patients with benign prostatic hyperplasia. Sex Med. 2020;8:205–13. https://doi.org/10.1016/j.esxm.2020.01.008
Mitidieri E, Cirino G, d’Emmanuele di Villa Bianca R, Sorrentino R. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. https://doi.org/10.1016/j.pharmthera.2020.107493
Kim S, Sung GT. Efficacy and safety of tadalafil 5 mg once daily for the treatment of erectile dysfunction after robot-assisted laparoscopic radical prostatectomy: a 2-year follow-up. Sex Med. 2018;6:108–14. https://doi.org/10.1016/j.esxm.2017.12.005
Nason GJ, McNamara F, Twyford M, O’Kelly F, White S, Dunne E, et al. Efficacy of vacuum erectile devices (VEDs) after radical prostatectomy: the initial Irish experience of a dedicated VED clinic. Int J Impot Res. 2016;28:205–8. https://doi.org/10.1038/ijir.2016.23
West M, Cordon BH, Ortega Y, Narus J, Mulhall JP. Pain associated with prostaglandin E1-containing intracavernosal injection medication is associated with poor erectile function recovery after radical prostatectomy. Andrology. 2024;13:1484–9. https://doi.org/10.1111/andr.13784
Kucuk EV, Tahra A, Bindayi A, Onol FF. Erectile dysfunction patients are more satisfied with penile prosthesis implantation compared with tadalafil and intracavernosal injection treatments. Andrology. 2016;4:952–6. https://doi.org/10.1111/andr.12237
Mullins BT, Basak R, Broughman JR, Chen RC. Patient-reported sexual quality of life after different types of radical prostatectomy and radiotherapy: analysis of a population-based prospective cohort. Cancer. 2019;125:3657–65. https://doi.org/10.1002/cncr.32288
Martínez-Jabaloyas JM, Gil-Salom M, Villamón-Fort R, Pastor-Hernández F, Martínez-García R, García-Sisamón F. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001;40:641–6. https://doi.org/10.1159/000049850.
Liu F, Yuan Y, Bai L, Yuan L, Li L, Liu J, et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021;43:101963. https://doi.org/10.1016/j.redox.2021.101963
Le DC, Ngo MT, Kuo YC, Chen SH, Lin CY, Ling TY, et al. Secretome from estrogen-responding human placenta-derived mesenchymal stem cells rescues ovarian function and circadian rhythm in mice with cyclophosphamide-induced primary ovarian insufficiency. J Biomed Sci. 2024;31:95. https://doi.org/10.1186/s12929-024-01085-8
Cao H, Chen M, Cui X, Liu Y, Liu Y, Deng S, et al. Cell-free osteoarthritis treatment with sustained-release of chondrocyte-targeting exosomes from umbilical cord-derived mesenchymal stem cells to rejuvenate aging chondrocytes. ACS Nano. 2023;17:13358–76. https://doi.org/10.1021/acsnano.3c01612
Luo JQ, Wang L, Liao ZQ, Lu BX, Luo CY, He HY, et al. Adipose stem cells ameliorate erectile dysfunction in diabetes mellitus rats by attenuating ferroptosis through NRP1 with SLC7A11 interaction. Free Radic Biol Med. 2025;232:40–55. https://doi.org/10.1016/j.freeradbiomed.2025.02.041
Khan N, Downey J, Sanz J, Kaufmann E, Blankenhaus B, Pacis A, et al. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell. 2020;183:752–70.e22. https://doi.org/10.1016/j.cell.2020.09.062
Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–31. https://doi.org/10.1016/j.stem.2020.09.014
You D, Jang MJ, Song G, Shin HC, Suh N, Kim YM, et al. Safety of autologous bone marrow-derived mesenchymal stem cells in erectile dysfunction: an open-label phase 1 clinical trial. Cytotherapy. 2021;23:931–8. https://doi.org/10.1016/j.jcyt.2021.06.001
Fu Q, Song L, Li J, Yi B, Huang Y, Zhang Z, et al. Biodegradable nano black phosphorus based SDF1-α delivery system ameliorates erectile dysfunction in a cavernous nerve injury rat model by recruiting endogenous stem/progenitor cells. J Nanobiotechnology. 2023;21:487. https://doi.org/10.1186/s12951-023-02238-x
Feng H, Liu Q, Deng Z, Li H, Zhang H, Song J, et al. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res Ther. 2022;13:450. https://doi.org/10.1186/s13287-022-03147-w
Yang J, Zhang Y, Zang G, Wang T, Yu Z, Wang S, et al. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology. 2018;6:498–509. https://doi.org/10.1111/andr.12483
Peng D, Yuan H, Liu T, Wang T, Reed-Maldonado AB, Kang N, et al. Smooth muscle differentiation of penile stem/progenitor cells induced by microenergy acoustic pulses in vitro. J Sex Med. 2019;16:1874–84. https://doi.org/10.1016/j.jsxm.2019.08.020
Ruan Y, Zhou J, Kang N, Reed-Maldonado AB, Tamaddon A, Wang B, et al. The effect of low-intensity extracorporeal shockwave therapy in an obesity-associated erectile dysfunction rat model. BJU Int. 2018;122:133–42. https://doi.org/10.1111/bju.14202
Shan HT, Zhang HB, Chen WT, Chen FZ, Wang T, Luo JT, et al. Combination of low-energy shock-wave therapy and bone marrow mesenchymal stem cell transplantation to improve the erectile function of diabetic rats. Asian J Androl. 2017;19:26–33. https://doi.org/10.4103/1008-682X.184271
He Y, Li F, Jiang P, Cai F, Lin Q, Zhou M, et al. Remote control of the recruitment and capture of endogenous stem cells by ultrasound for in situ repair of bone defects. Bioact Mater. 2022;21:223–38. https://doi.org/10.1016/j.bioactmat.2022.08.012
Xu Y, Xin H, Wu Y, Guan R, Lei H, Fu X, et al. Effect of icariin in combination with daily sildenafil on penile atrophy and erectile dysfunction in a rat model of bilateral cavernous nerves injury. Andrology. 2017;5:598–605. https://doi.org/10.1111/andr.12341
Zheng T, Zhang T, Zhang W, Lv K, Jia D, Yang F, et al. Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed Pharmacother. 2020;125:109888. https://doi.org/10.1016/j.biopha.2020.109888
Zhang J, Li S, Zhang S, Wang Y, Jin S, Zhao C, et al. Effect of icariside II and metformin on penile erectile function, histological structure, mitochondrial autophagy, glucose-lipid metabolism, angiotensin II and sex hormone in type 2 diabetic rats with erectile dysfunction. Sex Med. 2020;8:168–77. https://doi.org/10.1016/j.esxm.2020.01.006
Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–10. https://doi.org/10.1016/j.eururo.2011.07.061
Chen KK, Chan JY, Chang LS, Chen MT, Chan SH. Intracavernous pressure as an experimental index in a rat model for the evaluation of penile erection. J Urol. 1992;147:1124–8. https://doi.org/10.1016/s0022-5347(17)37500-6
Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. https://doi.org/10.1073/pnas.76.9.4350
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. https://doi.org/10.1186/s12974-020-1726-7
Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther. 2020;28:2203–19. https://doi.org/10.1016/j.ymthe.2020.06.026
Wang S, Du Y, Zhang B, Meng G, Liu Z, Liew SY, et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell. 2024;187:6152–64.e18. https://doi.org/10.1016/j.cell.2024.09.004
Xu H, Zhu Y, Hsiao AW, Xu J, Tong W, Chang L, et al. Bioactive glass-elicited stem cell-derived extracellular vesicles regulate M2 macrophage polarization and angiogenesis to improve tendon regeneration and functional recovery. Biomaterials. 2023;294:121998. https://doi.org/10.1016/j.biomaterials.2023.121998
Vaegler M, Lenis AT, Daum L, Amend B, Stenzl A, Toomey P, et al. Stem cell therapy for voiding and erectile dysfunction. Nat Rev Urol. 2012;9:435–47. https://doi.org/10.1038/nrurol.2012.111
Chen F, Zhang H, Wang Z, Ding W, Zeng Q, Liu W, et al. Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med. 2017;14:1084–94. https://doi.org/10.1016/j.jsxm.2017.07.005
Liang L, Shen Y, Dong Z, Gu X. Photoacoustic image-guided corpus cavernosum intratunical injection of adipose stem cell-derived exosomes loaded polydopamine thermosensitive hydrogel for erectile dysfunction treatment. Bioact Mater. 2021;9:147–56. https://doi.org/10.1016/j.bioactmat.2021.07.024
Kim SG, You D, Kim K, Aum J, Kim YS, Jang MJ, et al. Therapeutic effect of human mesenchymal stem cell-conditioned medium on erectile dysfunction. World J Mens Health. 2022;40:653–62. https://doi.org/10.5534/wjmh.210121
Luo C, Peng Y, Zhou X, Fan J, Chen W, Zhang H, et al. NLRP3 downregulation enhances engraftment and functionality of adipose-derived stem cells to alleviate erectile dysfunction in diabetic rats. Front Endocrinol (Lausanne). 2022;13:913296. https://doi.org/10.3389/fendo.2022.913296
Liu S, Jiang C, Hu J, Chen H, Han B, Xia S. Low-intensity pulsed ultrasound enhanced adipose-derived stem cell-mediated angiogenesis in the treatment of diabetic erectile dysfunction through the piezo-ERK-VEGF axis. Stem Cells Int. 2022;2022:6202842. https://doi.org/10.1155/2022/6202842
Chen Z, Han X, Ouyang X, Fang J, Huang X, Wei H. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics. 2019;9:6354–68. https://doi.org/10.7150/thno.34008
Moon HW, Kim IG, Kim MY, Jung AR, Park K, Lee JY. Erectile dysfunction treatment using stem cell delivery patch in a cavernous nerve injury rat model. Bioengineering (Basel). 2023;10:635. https://doi.org/10.3390/bioengineering10060635
Li M, Lei H, Xu Y, Li H, Yang B, Yu C, et al. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6:927–35. https://doi.org/10.1111/andr.12519
Xu Y, Guan R, Lei H, Li H, Wang L, Gao Z, et al. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med. 2014;11:2439–48. https://doi.org/10.1111/jsm.12636
Anatomy and physiology of erection: pathophysiology of erectile dysfunction. Int J Impot Res. 2003;15 Suppl 7:S5-8. https://doi.org/10.1038/sj.ijir.3901127.
Nandi S, Kumar P, Amin SA, Jha T, Gayen S. First molecular modelling report on tri-substituted pyrazolines as phosphodiesterase 5 (PDE5) inhibitors through classical and machine learning based multi-QSAR analysis. SAR QSAR Environ Res. 2021;32:917–39. https://doi.org/10.1080/1062936X.2021.1989721
Saikia Q, Hazarika A, Mishra R. A review on the pharmacological importance of PDE5 and its inhibition to manage biomedical conditions. Journal of Pharmacology and Pharmacotherapeutics. 2022;13:246–57. https://doi.org/10.1177/0976500X221129008
Lu L, Liu Y, Zhang X, Lin J. The therapeutic role of bone marrow stem cell local injection in rat experimental periodontitis. J Oral Rehabil. 2020;47(Suppl 1):73–82. https://doi.org/10.1111/joor.12843
Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. 2013;45:1083–6. https://doi.org/10.1016/j.biocel.2013.02.013
Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111–26. https://doi.org/10.1016/j.biotechadv.2018.03.011
Yang R, Fang F, Wang J, Guo H. Adipose-derived stem cells ameliorate erectile dysfunction after cavernous nerve cryoinjury. Andrology. 2015;3:694–701. https://doi.org/10.1111/andr.12047
Jeon SH, Zhu GQ, Bae WJ, Choi SW, Jeong HC, Cho HJ, et al. Engineered mesenchymal stem cells expressing stromal cell-derived factor-1 improve erectile dysfunction in streptozotocin-induced diabetic rats. Int J Mol Sci. 2018;19:3730. https://doi.org/10.3390/ijms19123730
Du L, Feng R, Ge S. PTH/SDF-1α cotherapy promotes proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2016;49:599–608. https://doi.org/10.1111/cpr.12286
Liang Q, Du L, Zhang R, Kang W, Ge S. Stromal cell-derived factor-1/Exendin-4 cotherapy facilitates the proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells in vitro and promotes periodontal bone regeneration in vivo. Cell Prolif. 2021;54:e12997. https://doi.org/10.1111/cpr.12997
Wang D, Lyu Y, Yang Y, Zhang S, Chen G, Pan J, et al. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022;140:610–24. https://doi.org/10.1016/j.actbio.2021.11.039
Rashidi NM, Scott MK, Scherf N, Krinner A, Kalchschmidt JS, Gounaris K, et al. In vivo time-lapse imaging shows diverse niche engagement by quiescent and naturally activated hematopoietic stem cells. Blood. 2014;124:79–83. https://doi.org/10.1182/blood-2013-10-534859
Hagedorn EJ, Perlin JR, Freeman RJ, Wattrus SJ, Han T, Mao C, et al. Transcription factor induction of vascular blood stem cell niches in vivo. Dev Cell. 2023;58:1037–51.e4. https://doi.org/10.1016/j.devcel.2023.04.007
Shen D, Li Z, Hu S, Huang K, Su T, Liang H, et al. Antibody-armed platelets for the regenerative targeting of endogenous stem cells. Nano Lett. 2019;19:1883–91. https://doi.org/10.1021/acs.nanolett.8b04970
Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. https://doi.org/10.1016/s0092-8674(02)00754-7
Shao J, Nie P, Yang W, Guo R, Ding D, Liang R, et al. An EPO-loaded multifunctional hydrogel synergizing with adipose-derived stem cells restores neurogenic erectile function via enhancing nerve regeneration and penile rehabilitation. Bioeng Transl Med. 2022;7:e10319 https://doi.org/10.1002/btm2.10319
Funding
This work was supported by grants from the National Natural Science Foundation of China (82171612, 82301820), the Science and Technology Projects in Guangzhou (2023A03J0789, 2025A04J5127), and the President Foundation of Nanfang Hospital, Southern Medical University (2021B014).
Author information
Authors and Affiliations
Contributions
HB Zhang and AY Wei designed experiments and helped write the manuscript. HY He, BL He and SH He performing data curation and conducting formal analysis. ZQ Liao, SB Duan, BX Lu, JQ Luo, HY He, BL He and SH He conducted the investigation. XC Zhou, HB Zhang and AY Wei provided resources. HB Zhang, SH He and AY Wei provided supervision. HY He and HB Zhang wrote the original draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All experimental protocols were approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University and were performed in accordance with the Declaration of Helsinki of the World Medical Association. All procedures were approved by the Medical Ethics Committee of Nangfang Hospital, Southern Medical University and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, H., Duan, S., He, B. et al. Adipose-derived stem cell therapy restores erectile function and promotes endogenous stem cell recruitment in a rat model of cavernous nerve injury. Int J Impot Res (2025). https://doi.org/10.1038/s41443-025-01162-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41443-025-01162-y