Fig. 1
From: Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts
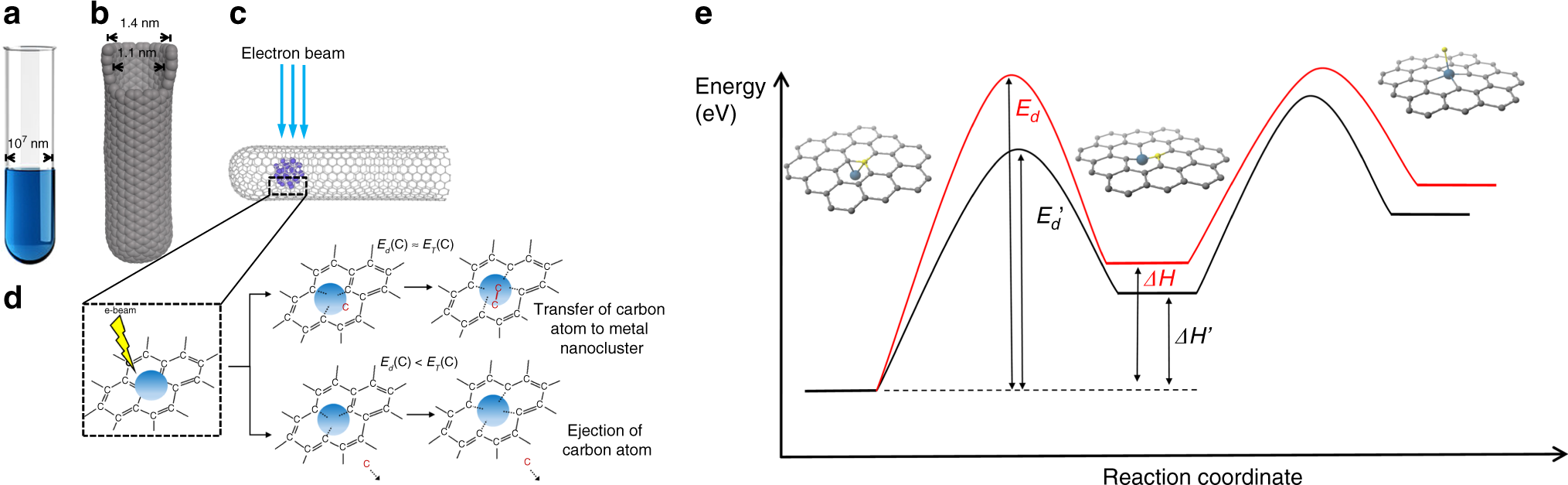
Fundamental principles of ChemTEM. In comparison to conventional glass test tubes (a), which have a diameter of \({\scriptstyle\sim}\)1 cm (107 nm), carbon nanotubes (b), possess a channel with a van der Waals diameter of 1.1 nm making them a suitable vessel for the entrapment of nano-sized samples of different chemical elements, that can also act as an effective nanoreactor provided that the nanotube structure is not defective and its inner channel is free of impurities. Our methodology enables the insertion of clusters of 20–60 metal atoms within nano test tubes (c) and investigation by aberration corrected high resolution TEM (AC-HRTEM) reveals the nanoclusters’ structures and chemical transformations at the atomic scale. The electron beam serves a dual purpose: some electrons (a tiny fraction) transfer a portion of their kinetic energy to the metal and carbon atoms, as described by the equation for ET above, which activates chemical transformations in the sample, whilst most of the electrons are transmitted through or scattered by the specimen generating two-dimensional images, the collation of which into time series enables the results of the induced transformations to be observed over time. d The presence of metal nanoclusters reduces the energy barrier for the displacement of a carbon atom (Ed) from the nanotube which can be either ejected, leading to a vacancy defect (bottom diagram) or converted into another carbon structure (top diagram), with both processes facilitated by the metal nanocluster (Supplementary Fig. 9). e A schematic energy profile of the formation of a vacancy defect illustrating a link between Ed and the enthalpy change (ΔH): the formation of metal-carbon (M–C) bonds between the metal nanocluster (represented by a single metal atom) and the dangling bonds of the defect make the defect more stable, thus lowering the energy threshold for defect formation (black curve) as compared to the same process in the absence of a metal nanocluster (red curve)
