Fig. 1: Electrochemical investigation on the transition between Zn and ZnO.
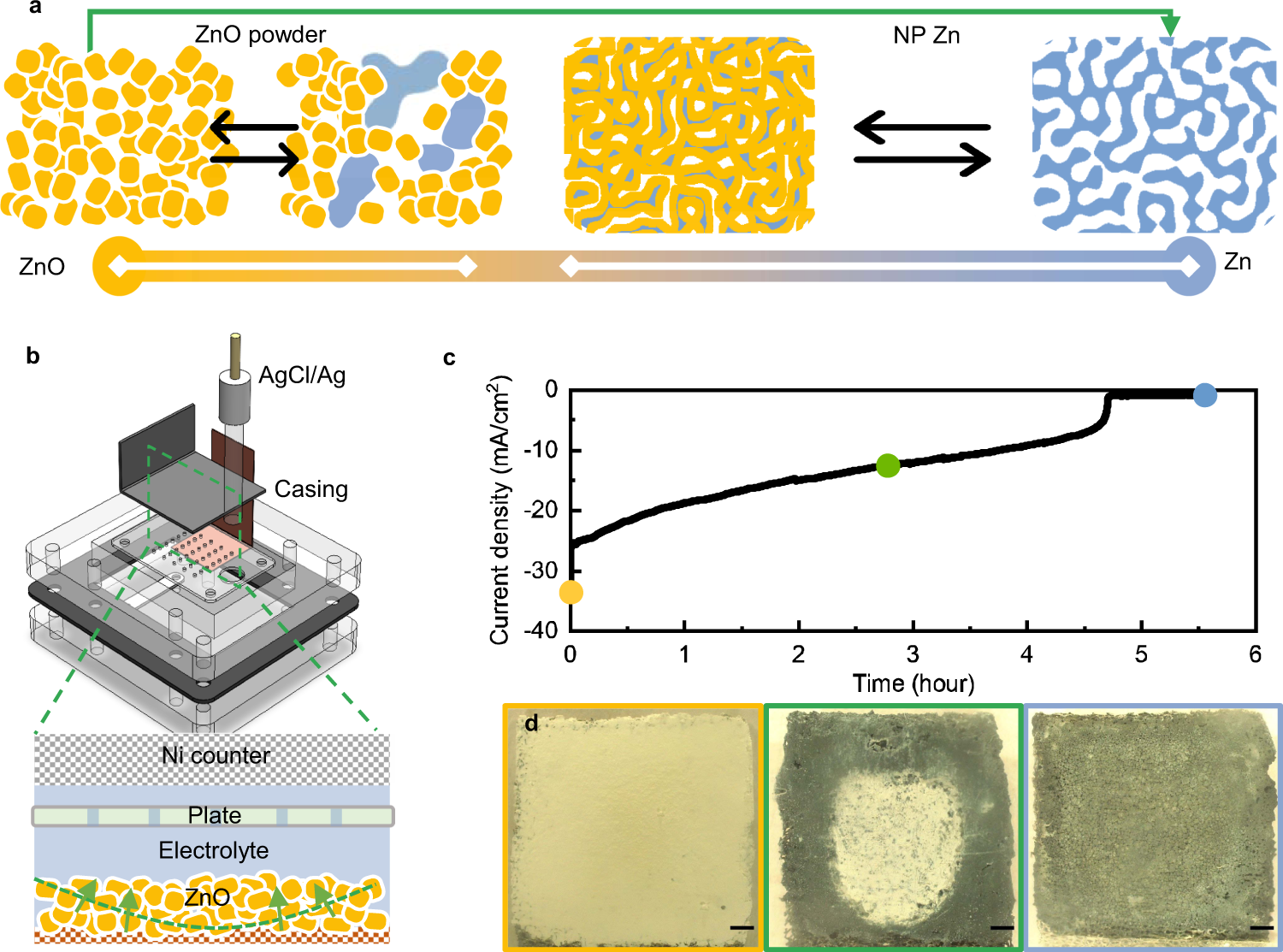
a An illustration of the different paths of transition with pure ZnO (yellow) on the left and pure Zn (blue) on the right. In a ZnO powder anode, the transition (black arrows on the left) is between the powder and clusters of Zn metal, whose non-uniform distribution limits the utilization of the Zn mass to <30% (a range marked with a white line at the bottom scale). Instead, the improved transition (the green arrow) takes the ZnO powder to NP Zn, which can then be cycled stably at >40% DoD (black arrows on the right), as highlighted similarly by the corresponding white line at the bottom scale. b The electrochemical cell designed for the improved transition through an electrochemical reduction, with the assembled electrolyte chamber highlighted at the bottom. The green arrows indicate the direction of the transition and the green dashed line the reaction front. c A typical chronoamperometric curve of the reduction. d Photos of the sample at the three stages of the reduction as labeled in c. The scale bars are 1 mm.
