Fig. 1: Protein-protein interaction between 14-3-3 and ChREBPα regulates β-cell fate.
From: Molecular glues of the regulatory ChREBP/14-3-3 complex protect beta cells from glucolipotoxicity
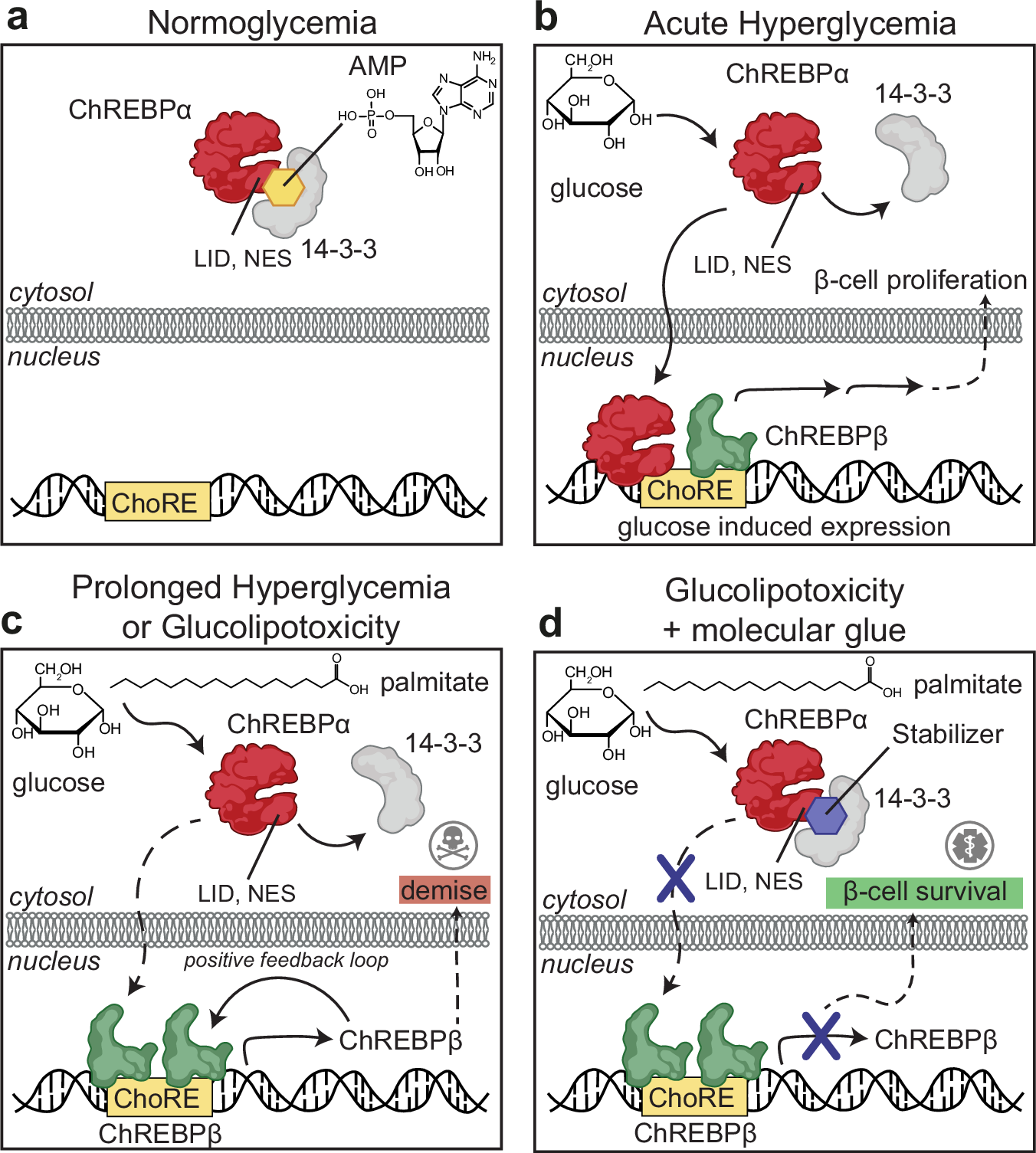
a Under normoglycemic conditions, ChREBPα remains mostly cytoplasmic by binding to 14-3-3. Some elements were made using BioRender.com. ChREBPα is one of very few phosphorylation-independent 14-3-3 partner proteins and binds via a pocket containing a phosphate or sulfate ion, ketone, or AMP. b In acute hyperglycemia, ChREBPα dissociates from 14-3-3 and transiently translocates into the nucleus where it binds multiple carbohydrate response elements (ChoREs) and promotes adaptive β-cell expansion. Some elements were made using BioRender.com. c In prolonged hyperglycemia or hyperglycemia combined with hyperlipidemia (glucolipotoxicity), ChREBPα initiates and maintains a feed-forward surge in ChREBPβ expression, leading to β-cell demise. Some elements were made using BioRender.com. d A novel class of molecular glue drugs specifically stabilize ChREBPα/14-3-3 interaction, prevent a surge of ChREBPβ expression in glucolipotoxicity, and protect β-cell identity and survival. Some elements were made using BioRender.com.
