Fig. 1: Assessing the structure-function relationship of anti-CD20 therapeutic antibodies in the cellular context using RESI microscopy.
From: Resolving the structural basis of therapeutic antibody function in cancer immunotherapy with RESI
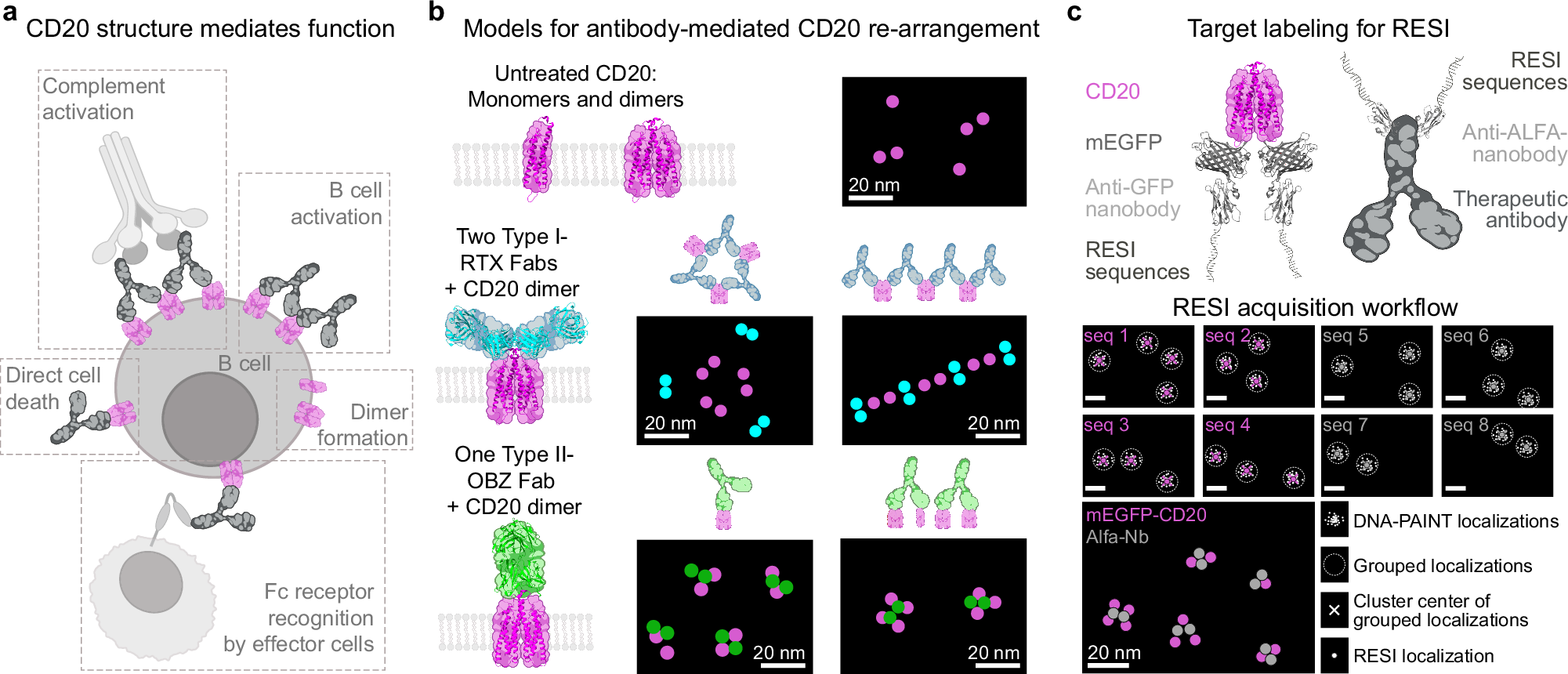
a The structural configuration of CD20 proteins and their interactions with therapeutic antibodies on the cell membrane influence the therapeutic efficacy of the antibodies. b CD20 proteins (magenta) in the membrane exist as a mixture of monomers and pre-formed dimers1. Rituximab (RTX) (cyan) can bind with 2 Fabs per CD20 dimer, thereby inducing higher-order arrangements of CD20 dimers2,3. However, the quantitative nature of the 3D cluster organization is still unknown. In contrast, Obinutuzumab (OBZ) (green) can bind with one Fab per single CD20 dimer, suggesting a terminal complex of up to CD20 tetramers by bridging two CD20 dimers. However, the resulting structural organization of CD20 – potentially forming monomers, dimers, trimers, or tetramers – remains to be fully elucidated. c We use two-target RESI super-resolution microscopy with four rounds per target to visualize and correlate the locations of mEGFP-tagged CD20 and ALFA-tagged therapeutic antibodies at single-protein resolution (sub-5 nm). CD20 is labeled in 1:1 stoichiometry and mAbs are labeled in a 2:1 stoichiometry. By performing eight consecutive DNA-PAINT imaging rounds and clustering localizations, we achieve precise RESI localizations (σ ≈ 0.6 nm) of the targets in their cellular context. Created with the help of BioRender (https://BioRender.com/y76v9f4 and https://BioRender.com/5575210).
