Fig. 1: Structures and absorption properties of the α and β subunits of human HbCO.
From: Direct observation of two-channel photodissociation of carbon monoxide from the hemoglobin subunits
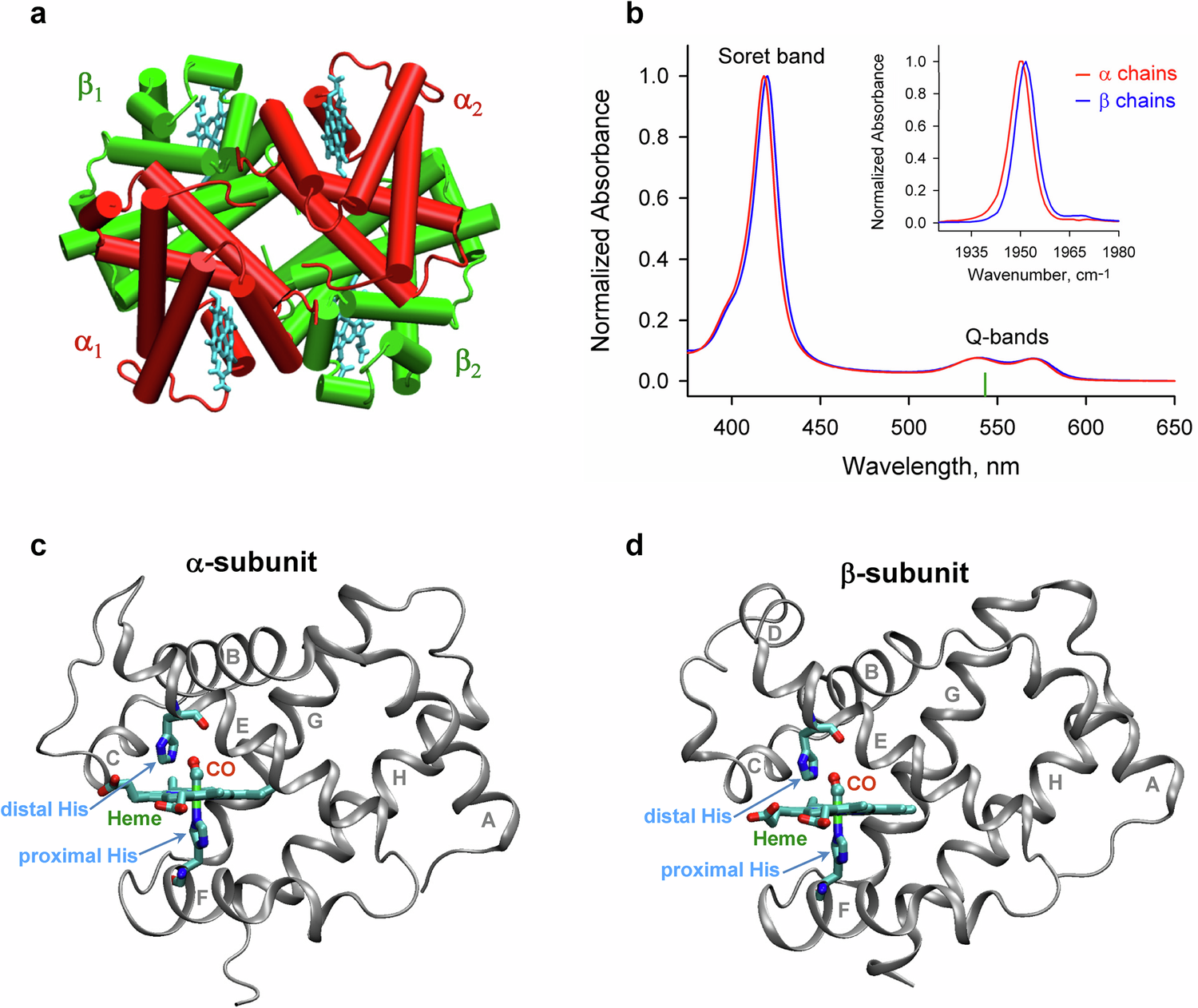
a The quaternary arrangement of the α and β subunits of HbCO (PDB entry 1BBB)7. The α and β subunits in red and green, respectively, are shown with the hemes in cyan. b Visible (the main figure) and mid-infrared absorption spectra (the inset) of the isolated carbonmonoxy α and β chains. The absorption spectra for the α and β chains are shown by the red and blue lines, respectively. The absorbance in the visible region is due to the heme group present in the proteins. The excitation wavelength, λexc = 543 nm, is indicated as the vertical green mark. In the inset, the FTIR spectra of the stretching bands of the heme-bound CO are shown. The visible absorption spectra for the α chains were repeated 7 times, and 8 times for the β chains. The mid-infrared absorption spectra were repeated 8 times for each sample. The structure of the α subunit (c) and the β subunit (d) of human HbCO (PDB entry 1BBB7). The heme group, the distal histidine as well as the proximal histidine, which links the heme iron to the F helix, are labeled and shown in sticks. The CO molecule bound to the heme iron in the distal heme pocket is labeled in red. Location of the CO binding site is marked by the heme-bound CO. Source data are provided as a Source Data file.
