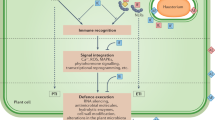
Abstract
Plants face constant microbial threats and have evolved highly effective immune systems characterized by inducible defence mechanisms. On recognizing microbial patterns and/or effectors, plants activate localized pattern-triggered immunity and/or effector-triggered immunity, which culminate in systemic acquired resistance—a broad-spectrum immune response that enhances protection throughout the plant. Systemic acquired resistance shares striking similarities with mammalian trained immunity, particularly in defence priming, which equips organisms with an enhanced capacity to respond to subsequent infections. This Review explores the cross-kingdom similarities between systemic acquired resistance and trained immunity, emphasizing their potential to transform agricultural practices and medical therapies. These insights present innovative opportunities for developing new plant-protection strategies, producing disease-resistant crops and optimizing vaccine approaches, while also highlighting critical knowledge gaps to inspire future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ross, A. F. Systemic acquired resistance induced by localized virus infections in plants. Virology 14, 340–358 (1961). This paper represents a seminal milestone in plant immunology, establishing the concept of systemic acquired resistance in plants.
Malamy, J., Carr, J. P., Klessig, D. F. & Raskin, I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250, 1002–1004 (1990).
Dean, R. A. & Kuć, J. Induced systemic protection in plants. Trends Biotechnol. 3, 125–129 (1985).
Ryals, J. A. et al. Systemic acquired resistance. Plant Cell 8, 1809–1819 (1996).
Fu, Z. Q. & Dong, X. Systemic acquired resistance: turning local infection into global defence. Annu. Rev. Plant Biol. 64, 839–863 (2013).
Mishina, T. E. & Zeier, J. The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol. 141, 1666–1675 (2006).
Hartmann, M. et al. Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173, 456–469 (2018).
Návarová, H., Bernsdorff, F., Döring, A.-C. & Zeier, J. Pipecolic acid, an endogenous mediator of defence amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24, 5123–5141 (2012).
Luna, E. et al. Next-generation systemic acquired resistance. Plant Physiol. 158, 844–853 (2012).
Jones, J. D. G. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006). This landmark paper elucidates the complex network of local defence mechanisms used by plants against pathogens, serving as a foundational reference for plant immunologists.
Jones, J. D., Staskawicz, B. J. & Dangl, J. L. The plant immune system: from discovery to deployment. Cell 187, 2095–2116 (2024). This article summarizes the current knowledge of extracellular and intracellular receptors that convert recognition of extracellular microbial patterns or intracellular pathogen-delivered virulence effectors into downstream signalling and local defence activation.
Padgett, H. S., Watanabe, Y. & Beachy, R. N. Identification of the TMV replicase sequence that activates the N gene-mediated hypersensitive response. Mol. Plant Microbe Interact. 10, 709–715 (1997).
Gruner, K. et al. Reprograming of plants during systemic acquired resistance. Front. Plant Sci. 4, 252 (2013).
Powers, J. et al. Next-generation mapping of the salicylic acid signalling hub and transcriptional cascade. Mol. Plant 17, 1558–1572 (2024). This study uncovers the NPR1 proxiome and its role in SA-induced plant immunity, revealing how NPR1 condensates orchestrate transcriptional cascades through chromatin remodelling and transcription factor recruitment, providing a blueprint for mapping signalling hubs in plant immunity.
Baum, S. et al. Isolation of open chromatin identifies regulators of systemic acquired resistance. Plant Physiol. 181, 817–833 (2019).
Sistenich, A. J. et al. Marker and readout genes for defence priming in Pseudomonas cannabina pv. alisalensis interaction aid understanding systemic immunity in Arabidopsis. Sci. Rep. 14, 3489 (2024).
Schwachtje, J., Fischer, A., Erban, A. & Kopka, J. Primed primary metabolism in systemic leaves: a functional systems analysis. Sci. Rep. 8, 216 (2018).
Zhang, H., Liu, Y., Zhang, X., Ji, W. & Kang, Z. A necessary considering factor for breeding: growth–defence trade-off in plants. Stress Biol. 3, 6 (2023).
Huot, B., Yao, J., Montgomery, B. L. & He, S. Y. Growth–defence trade-offs in plants: a balancing act to optimize fitness. Mol. Plant 7, 1267–1287 (2014).
Conrath, U. et al. Priming: getting ready for battle. Mol. Plant Microbe Interact. 19, 1062–1071 (2006).
Conrath, U. et al. Priming for enhanced defence. Annu. Rev. Phytopathol. 53, 97–119 (2015).
Spoel, S. H. & Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100 (2012).
Ausubel, F. M. Are innate immune signalling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979 (2005).
Roberts, D. A. Acquired resistance to tobacco mosaic virus transmitted to the progeny of hypersensitive tobacco. Virology 124, 161–163 (1983).
Slaughter, A. et al. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 158, 835–843 (2012).
Lamarck, J.-B. P. A. Philosophie Zoologique Vol. 1, ou Exposition des Considérations Relatives à l’Histoire Naturelle des Animaux (Dentu, 1809). In this book, Lamarck proposed that species could acquire new traits through environmental influences.
Pradeu, T. et al. The conceptual foundations of innate immunity: taking stock 30 years later. Immunity 57, 613–631 (2024).
Netea, M. G. et al. Trained immunity: a memory for innate host defence. Cell Host Microbe 9, 355–361 (2011).
Kurtz, J. & Franz, K. Evidence for memory in invertebrate immunity. Nature 425, 37–38 (2003).
Moret, Y. & Siva-Jothy, T. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 270, 2475–2480 (2003).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016). This is a pivotal contribution that elucidates the concept of trained immunity, revolutionizing our understanding of the innate immune system’s ability to mount enhanced responses to subsequent infections, with profound implications for human health and disease.
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Stewart, W. E., Gosser, L. B. & Lockart, R. Z. Priming: a non-anti-viral function of interferon. J. Virol. 7, 792–801 (1971).
Abreu, S. L., Bancroft, F. C. & Stewart, W. E. Interferon priming: effects on interferon messenger RNA. J. Biol. Chem. 254, 4114–4118 (1979).
Gifford, G. E. & Lohmann-Matthes, M.-L. Gamma interferon priming of mouse and human macrophages for induction of tumour necrosis factor production by bacterial lipopolysaccharide. J. Natl Cancer Inst. 78, 121–124 (1987).
Koerner, T. J., Adams, D. O. & Hamilton, T. A. Regulation of tumour necrosis factor (TNF) expression: interferon-ɣ enhances the accumulation of mRNA for TNF induced by polysaccharide in murine peritoneal macrophages. Cell. Immunol. 109, 437–443 (1987).
Hayes, M. P., Enterline, J. C., Gerrard, T. L. & Zoon, K. C. Regulation of interferon production by human monocytes: requirements for priming for lipopolysaccharide-induced production. J. Leukoc. Biol. 50, 176–181 (1991).
Hayes, M. P., Freeman, S. L. & Donnelly, R. P. IFN-ɣ priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and mRNA stability. Cytokine 7, 427–435 (1995).
Hayes, M. P., Wang, J. & Norcross, M. A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-ɣ of lipopolysaccharide-inducible p35 and p40 genes. Blood 86, 646–650 (1995).
Hayes, M. P. & Zoon, K. C. Priming of human monocytes for enhanced lipopolysaccharide responses: expression of alpha interferon, interferon regulatory factors, and tumour necrosis factor. Infect. Immun. 61, 3222–3227 (1993).
Hoffmann, H.-H., Schneider, W. M. & Rice, C. M. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 36, 124–138 (2015).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprograming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Vuscan, P. et al. Potent induction of trained immunity by Saccharomyces cerevisiae β-glucans. Front. Immunol. 15, 1323333 (2024).
Wang, G. et al. β-Glucan induces trained immunity to promote anti-viral activity by activating TBK1. Viruses 15, 1204 (2023).
Van Aubel, G., Cambier, P., Dieu, M. & Van Cutsem, P. Plant immunity induced by COS-OGA elicitor is a cumulative process that involves salicylic acid. Plant Sci. 247, 60–70 (2016).
Grebenciucova, E. & VanHaerents, S. Interleukin 6: at the interface of human health and disease. Front. Immunol. 14, 1255533 (2023).
Ciarlo, E. et al. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 222, 1869–1881 (2020).
Katzmarski, N. et al. Transmission of trained immunity and heterologous resistance to infection across generations. Nat. Immunol. 22, 1382–1390 (2021).
Métraux, J. P. et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250, 1004–1006 (1990).
Shulaev, V., Leon, J. & Raskin, I. Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell 7, 1691–1701 (1995).
Shulaev, V., Silverman, P. & Raskin, I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385, 718–721 (1997).
Park, S.-W., Kaimoyo, E., Kumar, D., Mosher, S. & Klessig, D. F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116 (2007).
Truman, W., Bennett, M. H., Kubigsteltig, I., Turnbull, C. & Grant, M. Arabidopsis systemic immunity uses conserved defence signalling pathways and is mediated by jasmonates. Proc. Natl Acad. Sci. USA 104, 1075–1080 (2007).
Jung, H. W., Tschaplinski, T. J., Wang, L., Glazebrook, J. & Greenberg, J. T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
Chaturvedi, R. et al. An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 71, 161–172 (2012).
Li, Q. et al. N-hydroxypipecolic acid triggers systemic acquired resistance through extracellular NAD(P). Nat. Commun. 14, 6848 (2023).
Chen, Y.-C. et al. N-hydroxypipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, E4920–E4929 (2018).
Shine, M. B. et al. Phased small RNA–mediated systemic signalling in plants. Sci. Adv. 8, eabm8791 (2022).
Alvarez, M. E. et al. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784 (1998).
Cao, L. et al. H2O2 sulfenylates CHE, linking local infection to the establishment of systemic acquired resistance. Science 385, 1211–1217 (2024).
Maldonado, A. M., Doerner, P., Dixon, R. A., Lamb, C. J. & Cameron, R. K. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419, 399–403 (2002).
Chanda, B. et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43, 421–427 (2011).
Riedlmeier, M. et al. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 29, 1440–1459 (2017).
Wenig, M. et al. Systemic acquired resistance networks amplify airborne defence cues. Nat. Commun. 10, 3813 (2019).
Brambilla, A. et al. Immunity-associated volatile emissions of β-ionone and nonanal propagate defence responses in neighbouring barley plants. J. Exp. Bot. 73, 615–630 (2022).
Brambilla, A. et al. Pipecolic acid synthesis is required for systemic acquired resistance and plant-to-plant-induced immunity in barley. J. Exp. Bot. 19, 3033–3046 (2013).
Delaney, T. P. et al. A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250 (1994).
Šestan, M. et al. An IFNγ-dependent immune–endocrine circuit lowers blood glucose to potentiate the innate anti-viral immune response. Nat. Immunol. 25, 981–993 (2024).
Fanucchi, S., Dominguez-Andrea, J., Joosten, L. A. B., Netea, M. G. & Mhlanga, M. M. The intersection of epigenetics and metabolism in trained immunity. Immunity 54, 32–43 (2021).
Bhargavi, G. & Subbian, S. The causes and consequences of trained immunity in myeloid cells. Front. Immunol. 15, 1365127 (2024).
Wang, D., Weaver, N. D., Kesarwani, M. & Dong, X. Induction of protein secretory pathway is required for systemic acquired resistance. Science 308, 1036–1040 (2005).
Kohler, A., Schwindling, S. & Conrath, U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol. 128, 1046–1056 (2002).
Zavaliev, R. & Dong, X. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 84, 131–141 (2024).
Chhillar, H., Nguyen, H. H., Yeh, P.-M., Jones, J. D. G. & Ding, P. Modular mechanisms of immune priming and growth inhibition mediated by plant effector-triggered immunity. Cell Rep. 44, 115394 (2025).
Wang et al. The truncated TNL receptor TN2-mediated immune responses require ADR1 function. Plant J. 108, 672–689 (2021).
Pick, T., Jaskiewicz, M., Peterhänsel, C. & Conrath, U. Heat shock factor HsfB1 primes gene transcription and systemic acquired resistance in Arabidopsis. Plant Physiol. 159, 52–55 (2012).
Pajerowska-Mukhatar, K. M. et al. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defence transition. Curr. Biol. 22, 103–112 (2012).
Van Anken, E. et al. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18, 243–253 (2003).
Beckers, G. J. M. et al. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21, 944–953 (2009). This study identifies MAPK3 and MAPK6 as key latent signalling components accumulated during SAR, revealing a molecular basis for defence priming against biotic and abiotic stress.
Tateda, C. et al. Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signalling. Plant Cell 26, 4171–4187 (2014). This study elucidates the crucial role of salicylic acid in enhancing microbial pattern receptor levels in primed Arabidopsis plants, shedding light on an intricate mechanism of plant defence priming.
Gómez-Gómez, L. & Boller, T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5, 1003–1011 (2000).
Chinchilla, D. et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500 (2007).
Boller, T. & Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009).
Mithoe, S. C. & Menke, F. L. H. Regulation of pattern recognition receptor signalling by phosphorylation and ubiquitination. Curr. Opin. Plant Biol. 45, 162–170 (2018).
Gong, B.-Q. et al. Cross-microbial protection via priming a conserved immune co-receptor through juxtamembrane phosphorylation in plants. Cell Host Microbe 26, 810–822 (2019).
Sirén, J., Pirhonen, J., Julkunen, I. & Matikainen, S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J. Immunol. 174, 1932–1937 (2005).
Schroder, K., Sweet, M. J. & Hume, D. A. Signal integration between IFN-γ and TLR signalling pathways in macrophages. Immunobiology 211, 511–524 (2006). This study reveals that natural killer cells exhibit adaptive immune features, challenging the traditional distinction between innate and adaptive immunity.
Badeaux, A. I. & Shi, Y. Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 14, 211–224 (2013).
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Roudier, F. et al. Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J. 30, 1928–1938 (2011).
Ng, M. K. & Cheung, P. A brief histone in time: understanding the combinatorial functions of histone PTMs in the nucleosome context. Biochem. Cell Biol. 94, 33–42 (2016).
Peña, P. V. et al. Molecular mechanism of histone H3K4me3 recognition by plant homeo-domain of ING2. Nature 442, 100–103 (2006).
Kanno, T. et al. Selective recognition of acetylated histones by bromo-domain proteins visualized in living cells. Mol. Cell 13, 33–43 (2004).
Eberharter, A. & Becker, P. B. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 3, 224–229 (2002).
He, G., Elling, A. A. & Deng, X. W. The epigenome and plant development. Annu. Rev. Plant Biol. 62, 411–435 (2011).
Jaskiewicz, M., Conrath, U. & Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 12, 50–55 (2011). This paper reports that chromatin modifications serve as a molecular memory for SAR, enabling plants to mount faster and stronger defence responses upon reinfection.
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Zhu, D. et al. Distinct chromatin signatures in the Arabidopsis male gametophyte. Nat. Genet. 55, 706–720 (2013).
Keating, S. T. et al. The Set7 lysine methyltransferase regulates plasticity in oxidative phosphorylation necessary for trained immunity induced by β-glucan. Cell Rep. 31, 107548 (2020).
Henikoff, S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat. Rev. Genet. 9, 15–26 (2008).
Gaulton, K. J. et al. A map of open chromatin in human pancreatic islets. Nat. Genet. 42, 255–259 (2010).
Buenrosto, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Kleinnijenhuis, J. et al. Bacille Calmette–Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 (2018). This study reveals that BCG reprogrammes hematopoietic stem cells in the bone marrow, leading to sustained innate immune memory and enhanced macrophage-mediated protection against tuberculosis, offering new insights for vaccine development.
Pavet, V. et al. Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by Pseudomonas syringae. Mol. Plant Microbe Interact. 19, 577–587 (2006).
Ptashne, M. Faddish stuff: epigenetics and the inheritance of acquired characteristics. FASEB J. 27, 1–2 (2013).
Baird, L. M., Berndsen, C. E. & Monroe, J. D. Malate dehydrogenase in plants: evolution, structure, and a myriad of functions. Essays Biochem. 68, 221–233 (2014).
Pracharoenwattana, I. et al. Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J. 62, 785–795 (2010).
Fernie, A. R., Carrari, F. & Sweetlove, L. J. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 7, 254–261 (2004).
Zamboni, N., Fendt, S.-M., Rühl, M. & Sauer, U. 13C-based metabolic flux analysis. Nat. Protoc. 4, 878–892 (2009).
Balmer, A., Pastor, V., Glauser, G. & Mauch-Mani, B. Tricarboxylates induce defence priming against bacteria in Arabidopsis thaliana. Front. Plant Sci. 9, 1221 (2018).
Zimmerli, L., Jakab, G., Métraux, J.-P. & Mauch-Mani, B. Potentiation of pathogen-specific defence mechanisms in Arabidopsis by β-aminobutyric acid. Proc. Natl Acad. Sci. USA 97, 12920–12925 (2000).
Jakab, G. et al. β-aminobutyric acid-induced resistance in plants. Eur. J. Plant Pathol. 107, 29–37 (2001).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immune-metabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Zhang, X. & Mou, Z. Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. Plant J. 57, 302–312 (2009).
Wang, C. et al. Extracellular pyridine nucleotides trigger plant systemic immunity through a lectin receptor kinase/BAK1 complex. Nat. Commun. 10, 4810 (2019).
Pétriacq, P. et al. NAD acts as an integral regulator of multiple defence layers. Plant Physiol. 172, 1465–1479 (2016).
Adriouch, S. et al. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J. Immunol. 179, 186–194 (2007).
Minhas, P. S. et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Hong, S. et al. Differential regulation of P2X7 receptor activation by extracellular NAD and ecto-ARTs in murine macrophages and T cells. J. Immunol. 183, 578–592 (2009).
Lee, H. C. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J. Biol. Chem. 287, 31633–31640 (2012).
Cheng, S. C. et al. mTOR- and HIF-1a–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Imai, S.-i & Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 (2014).
Acevedo, O. A. et al. Molecular and cellular mechanisms modulating trained immunity by various cell types in response to pathogen encounter. Front. Immunol. 12, 745332 (2021).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293 (2010).
Heinrich, P. C., Behrmann, I., Müller-Newen, G., Schaper, F. & Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–324 (1998).
Reeh, H. et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: fusing experimental insights and dynamic modelling. Cell Commun. Signal. 17, 46 (2019).
Hu, X. & Ivashkiv, L. B. Cross-regulation of signalling and immune responses by IFN-ɣ and STAT1. Immunity 31, 539–550 (2009).
Wu, F. et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578, 577–581 (2020).
Ding, Y. et al. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454–1467 (2018).
Spoel, S. H. & Dong, X. Salicylic acid in plant immunity and beyond. Plant Cell 36, 1451–1464 (2024).
Nair, A. et al. N-hydroxypipecolic acid-induced transcription requires the salicylic acid signalling pathway at basal SA levels. Plant Physiol. 187, 2803–2819 (2021).
Bauerle, P. A. & Baltimore, D. NF-kappa B: ten years after. Cell 87, 13–20 (1996).
Vo, N. & Goodman, R. H. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276, 13505 (2001).
Ryals, J. et al. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell 9, 425–439 (1997).
Kesanakurti, D., Sareddy, G. R., Babu, B. P. & Kirti, P. B. Mustard NPR1, a mammalian I-kappa-B homologue inhibits NF-kappa B activation in human GBM cell lines. Biochem. Biophys. Res. Commun. 390, 427–433 (2009).
Zhang, N. et al. Early-life exercise induces immune-metabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice. Nat. Commun. 15, 3103 (2024).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Shi, L., Wu, Y. & Sheen, J. TOR signalling in plants: conservation and innovation. Development 145, dev160887 (2018).
De Vleesschauwer, D. et al. Target of rapamycin signalling orchestrates growth–defence trade-offs in plants. N. Phytol. 217, 305–319 (2018).
Aznar, N. R., Consolo, V. F., Salerno, G. L. & Martinez-Noel, G. M. A. TOR signalling downregulation increases resistance to the cereal killer Fusarium graminearum. Plant Signal. Behav. 13, e1414120 (2018).
Buffen, K. et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 10, e1004485 (2014).
Fanucchi, S. et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 51, 138–150 (2019).
Yu, Y. et al. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol. J. 18, 679–690 (2020).
Huang, J., Zhou, W., Zhang, X. & Li, Y. Roles of long non-coding RNAs in plant immunity. PLoS Pathog. 19, e1011340 (2023).
López Sánchez, A., Stassen, J. H. M., Furci, L., Smith, L. M. & Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 88, 361–374 (2016).
Caldwell, B. A., Wu, Y., Wang, J. & Li, L. Altered DNA methylation underlies monocyte dysregulation and immune exhaustion memory in sepsis. Cell Rep. 43, 113894 (2024).
Furci, L. et al. Identification and characterization of hypomethylated DNA loci controlling quantitative resistance in Arabidopsis. eLife 8, e40655 (2019).
Lee, S. C. et al. Chromatin remodelling of histone H3 variants by DDM1 underlies epigenetic inheritance of DNA methylation. Cell 186, 4100–4116 (2023).
Lee, S. et al. DDM1-mediated gene body DNA methylation is associated with inducible activation of defence-related genes in Arabidopsis. Genome Biol. 24, 106 (2023).
Cambiagno, D. A., Torres, J. R. & Alvarez, M. E. Convergent epigenetic mechanisms avoid constitutive expression of immune receptor gene subsets. Front. Plant Sci. 12, 703667 (2012).
Ando, S. et al. Priming for enhanced ARGONAUTE2 activation accompanies induced resistance to cucumber mosaic virus in Arabidopsis thaliana. Mol. Plant Pathol. 22, 19–30 (2021).
Wilkinson, S. W. et al. Long-lasting memory of jasmonic acid-dependent immunity requires DNA demethylation and ARGONAUTE1. Nat. Plants 9, 81–95 (2013).
Ugarte, P. B. et al. Argonaute proteins confer immunity in all domains of life. Curr. Opin. Microbiol. 74, 102313 (2023).
Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (European Commission, 2020).
Martinez-Medina, A. et al. Recognizing plant defence priming. Trends Plant Sci. 21, 818–822 (2016).
Leadbeater, A. & Staub, T. in Induced Resistance for Plant Defence: A Sustainable Approach to Crop Protection 2nd edn (eds Walters, D. R. et al.) 300–315 (Wiley, 2014).
Cao, H., Li, X. & Dong, X. Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl Acad. Sci. USA 95, 6531–6536 (1998).
Zhou, J. et al. Trained immunity contributes to the prevention of Mycobacterium tuberculosis infection, a novel role of autophagy. Emerg. Microbes Infect. 10, 578–588 (2021).
Sohrabi, Y. et al. Trained immunity as a novel approach against COVID‐19 with a focus on Bacillus Calmette–Guérin vaccine: mechanisms, challenges and perspectives. Clin. Transl. Immunol. 9, e1228 (2020).
Wang, T. et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat. Immunol. 24, 423–438 (2023).
Lérias, J. R. et al. Trained immunity for personalized cancer immunotherapy: current knowledge and future opportunities. Front. Microbiol. 10, 2924 (2020).
Usher, N. T. et al. Association of BCG vaccination in childhood with subsequent cancer diagnoses: a 60-year follow-up of a clinical trial. JAMA Netw. Open 2, e1912014 (2019).
Funes, S. C. et al. Trained immunity contribution to autoimmune and inflammatory disorders. Front. Immunol. 13, 868343 (2022).
Badii, M. et al. Trained immunity and inflammation in rheumatic diseases. Joint Bone Spine 89, 105364 (2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author is a cofounder of AgPrime GmbH and a scientific advisor to Ascribe Bioscience Inc. and Südzucker AG. These affiliations had no influence on the content, analysis or conclusions of this Review.
Peer review
Peer review information
Nature Plants thanks Nicolas Cecchini, Daniel Klessig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Conrath, U. Cross-kingdom mechanisms of trained immunity in plant systemic acquired resistance. Nat. Plants 11, 1993–2005 (2025). https://doi.org/10.1038/s41477-025-02119-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02119-1