Abstract
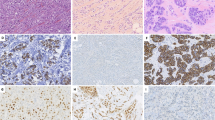
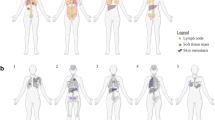
While primary invasive lobular carcinoma (ILC) is well characterized, metastatic ILC remains understudied. Within the post-mortem tissue donation programs, UPTIDER (Belgium) and Hope for Others (USA), we first aimed to explore intra-patient heterogeneity of key prognostic and predictive markers (stromal tumor-infiltrating lymphocytes (sTIL), estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and KI67). Secondly, we compared detection of the metastases by pathology on autopsy samples versus pre-mortem imaging. In total, 306 metastases from 12 patients were collected at autopsy (median: 27 per patient). Both primary tumors (n = 15) and metastases (n = 232) had low sTIL levels, with a median of 2% (range: 0.67–6.67%) and 0.67% (range: 0–13.33%), respectively. Regression models showed lower ER- and PR-expression in metastases (respectively, n = 265 and n = 64) compared to primary tumors (both p < 0.01). KI67 was significantly higher in metastases (n = 262, p = 0.02). HER2-low metastases were found in all but one patient although in varying proportion of metastases (range: 7.5–100%). Central radiology and pathology review had a median concordance of 78% at organ level (range: 33.33–100%) and 71% at patient level (range: 55.88-85.29%). Our findings suggest that a single metastatic biopsy has great limitations to guide treatment and that more adequate methods are needed to detect and monitor ILC metastases.
Similar content being viewed by others
Data availability
The histopathological data underlying this article will be available in a CodeOcean repository with the following https://doi.org/10.24433/CO.7065184.v1.
References
Reed, M. E. M. C., Kutasovic, J. R., Lakhani, S. R. & Simpson, P. T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and’omics. Breast Cancer Research 17 https://doi.org/10.1186/s13058-015-0519-x (2015).
Pestalozzi, B. C. et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: Combined results of 15 International Breast Cancer Study Group clinical trials. J. Clin. Oncol. 26, 3006–3014 (2008).
Sastre-Garau, X. et al. Infiltrating Lobular Carcinoma of the Breast Clinicopathologic Analysis of 975 Cases with Reference to Data on Conservative Therapy and Metastatic Patterns. https://doi.org/10.1002/(SICI)1097-0142(19960101)77:1.
He, H., Gonzalez, A., Robinson, E. & Yang, W. T. Distant Metastatic Disease Manifestations in Infiltrating Lobular Carcinoma of the Breast. 202, 1140–1148 (2014).
Mathew, A. et al. Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast. Geburtshilfe Frauenheilkd. 77, 660–666 (2017).
Mollica, L., Leli, C., Puglisi, S., Sardi, S. & Sottotetti, F. Leptomeningeal carcinomatosis and breast cancer: a systematic review of current evidence on diagnosis, treatment and prognosis. Drugs Context 10, 1–23 (2021).
Tham, Y. L., Sexton, K., Kramer, R., Hilsenbeck, S. & Elledge, R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 107, 696–704 (2006).
Klebe, M. et al. Frequent Molecular Subtype Switching and Gene Expression Alterations in Lung and Pleural Metastasis From Luminal A–Type Breast Cancer. JCO Precis Oncol 848–859 (2020) https://doi.org/10.1200/PO.19.00337/SUPPL_FILE/DS_PO.19.00337-3.PDF.
Trillo, P. et al. Evolution of biological features of invasive lobular breast cancer: Comparison between primary tumour and metastases. Eur. J. Cancer 185, 119–130 (2023).
Christgen, M. et al. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathology Research Practice 212, 583–597 (2016).
De Schepper, M. et al. Integration of pathological criteria and immunohistochemical evaluation for invasive lobular carcinoma diagnosis: recommendations from the European Lobular Breast Cancer Consortium. Modern Pathology 100497 (2024) https://doi.org/10.1016/J.MODPAT.2024.100497.
Van Baelen, K. et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol https://doi.org/10.1016/J.ANNONC.2022.05.006 (2022).
Bhaludin, B. N. et al. A review on the added value of whole-body MRI in metastatic lobular breast cancer. Eur. Radio. 32, 6514–6525 (2022).
Ulaner, G. A. 16α-18F-fluoro-17β-Fluoroestradiol (FES): Clinical Applications for Patients With Breast Cancer. Semin Nucl. Med 52, 574–583 (2022).
Eshet, Y. et al. The Role of 68Ga-FAPI PET/CT in Detection of Metastatic Lobular Breast Cancer. Clin. Nucl. Med 48, 228–232 (2023).
Schuster, D. et al. 18F-Fluciclovine and 68Ga-PSMA-11 PET/CT for Detection of Invasive Lobular Breast Cancer: Interim Report from an Exploratory Trial. J. Nucl. Med. 63, 2592–2592 (2022).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer ✰. Ann. Oncol. 32, 1475–1495 (2021).
Geukens, T. et al. Research autopsy programmes in oncology: shared experience from 14 centres across the world. J. Pathol. 263, 150–165 (2024).
Geukens, T. et al. Rapid autopsies to enhance metastatic research: the UPTIDER post-mortem tissue donation program. NPJ Breast Cancer 10, (2024).
Ferlicot, S. et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur. J. Cancer 40, 336–341 (2004).
Li, C. I., Uribe, D. J. & Daling, J. R. Clinical characteristics of different histologic types of breast cancer. Br. J. Cancer 93, 1046–1052 (2005).
Roh, S. & Xu, L. Breast Cancer Metastatic to Gluteus Maximus: A Case Report. J. Breast Dis. 11, 30–33 (2023).
Karjol, U., Jonnada, P., Cherukuru, S. & Chandranath, A. Bladder Metastasis from Breast Cancer: A Systematic Review. Cureus 12, (2020).
Richard, F. et al. Characterization of stromal tumor-infiltrating lymphocytes and genomic alterations in metastatic lobular breast cancer. Clin. Cancer Res. 26, 6254–6265 (2020).
Szekely, B. et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 29, 2232–2239 (2018).
Hutchinson, K. E. et al. Comprehensive Profiling of Poor-Risk Paired Primary and Recurrent Triple-Negative Breast Cancers Reveals Immune Phenotype Shifts. Clin. Cancer Res 26, 657 (2020).
Voorwerk, L. et al. PD-L1 blockade in combination with carboplatin as immune induction in metastatic lobular breast cancer: the GELATO trial. Nat. Cancer 4, 535 (2023).
Gomez-Fernandez, C. et al. Immunohistochemically determined estrogen receptor phenotype remains stable in recurrent and metastatic breast cancer. Am. J. Clin. Pathol. 130, 879–882 (2008).
Lindström, L. S. et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 30, 2601–2608 (2012).
Hanker, A. B., Sudhan, D. R. & Arteaga, C. L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell https://doi.org/10.1016/j.ccell.2020.03.009 (2020).
Hsu, R. et al. Evaluation of markers of immunity in different metastatic immune microenvironments suggests more suppression within breast to liver metastases in breast cancer. Breast Cancer Res Treat. 206, 245 (2024).
Hilbers, F. et al. Characterization of the immune microenvironment in matched primary and metastatic breast cancer lesions from the AURORA study: BIG 14-01. J. Clin. Oncol. 41, 1009–1009 (2023).
Rye, I. H. et al. Breast cancer metastasis: immune profiling of lymph nodes reveals exhaustion of effector T cells and immunosuppression. Mol. Oncol. 16, 88–103 (2022).
Grinda, T. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter French ESME cohort. J Breast Cancer 7, 1–9 (2021).
Aurilio, G. et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 50, 277–289 (2014).
Schrijver, W. A. M. E. et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J. Natl. Cancer Inst. 110, 568–580 (2018).
Modi, S. et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 387, 9–20 (2022).
Geukens, T. et al. Intra-patient and inter-metastasis heterogeneity of HER2-low status in metastatic breast cancer. Eur. J. Cancer 188, 152–160 (2023).
Inari, H. et al. Clinicopathological and prognostic significance of Ki-67 immunohistochemical expression of distant metastatic lesions in patients with metastatic breast cancer. Breast Cancer 24, 748–755 (2017).
Deutsch, T. M. et al. Relationship of Ki-67 index in biopsies of metastatic breast cancer tissue and circulating tumor cells (CTCs) at the time of biopsy collection. Arch. Gynecol. Obstet. 309, 235–248 (2024).
Falato, C. et al. Ki67 measured in metastatic tissue and prognosis in patients with advanced breast cancer. Breast Cancer Res Treat. 147, 407–414 (2014).
Zels, G. et al. Histopathological Insights into Metastatic Breast Cancer Gained From Rapid Autopsies. Lab Invest 105, 104202 (2025).
Cardoso, F. et al. 6th and 7th International consensus guidelines for the management of advanced breast cancer (ABC guidelines 6 and 7). Breast 76, 103756 (2024).
Dowling, G. P. et al. Receptor Discordance in Metastatic Breast Cancer; a review of clinical and genetic subtype alterations from primary to metastatic disease. Breast Cancer Res Treat. 207, 471–476 (2024).
Zattarin, E. et al. Hormone Receptor Loss in Breast Cancer: Molecular Mechanisms, Clinical Settings, and Therapeutic Implications. Cells 9, (2020).
Weaver, O. & Yang, W. Imaging of Breast Cancers With Predilection for Nonmass Pattern of Growth: Invasive Lobular Carcinoma and DCIS—Does Imaging Capture It All?. Am. J. Roentgenol. 215, 1504–1511 (2020).
Zugni, F. et al. The added value of whole-body magnetic resonance imaging in the management of patients with advanced breast cancer. PLoS One 13, (2018).
Oesterreich, S. et al. International survey on invasive lobular breast cancer identifies priority research questions. NPJ Breast Cancer 10, 61 (2024).
Chang, A. CC. et al. Hope for Others: Research Results from the University of Pittsburgh Rapid Autopsy Program for Breast Cancer. Breast Cancer Res 27, 111 (2025).
Saini, K. S. & Twelves, C. Determining lines of therapy in patients with solid cancers: a proposed new systematic and comprehensive framework. Br. J. Cancer 125, 155–163 (2021).
Kos, Z. et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 6, (2020).
El Bairi, K. et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 7, (2021).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259 (2015).
Leduc, S. et al. Histopathological growth patterns and tumor-infiltrating lymphocytes in breast cancer liver metastases. NPJ Breast Cancer 9, (2023).
Arber, D. A. Effect of prolonged formalin fixation on the immunohistochemical reactivity of breast markers. Appl. Immunohistochemistry Mol. Morphol. 10, 183–186 (2002).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/ college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Acknowledgements
We would like to thank all patients participating in these programs, as well as their families who supported them. We thank healthcare staff, researchers and patient advocates who have been supportive of this project from the very beginning. UPTIDER was funded by the Klinische Onderzoeks- en Opleidingsraad (KOOR) of University Hospitals Leuven (Uitzonderlijke Financiering 2020) and C1 of KU Leuven (C14/21/114). This project was funded by the Belgian Foundation against Cancer (C/2022/2046). K.V.B. is funded by a Conquer Cancer – Lobular Breast Cancer Alliance Young Investigator Award for Invasive Lobular Carcinoma Research, supported by Lobular Breast Cancer Alliance. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology® or Conquer Cancer®, or Lobular Breast Cancer Alliance. K.V.B., M.D.S., J.V.C., K.B. are funded by the KU Leuven fund Nadine de Beauffort; T.G., F.R., GF and HW by the Research Foundation Flanders (FWO), MDS and MM by the Luxemburg Cancer Foundation, MM and H-LN by the European Research Council (ERC, FAT-BC 101003153), K.V.B., MM, FR by the Breast Cancer Research Foundation, and SL by the Belgian Cancer Foundation (2020-103). The HfO program has been supported by the Magee Womens Research Institute and Foundation, and Susan G Komen Foundation. This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by award P30CA047904. Work performed in the Pitt Biospecimen Core (RRID:SCR_025229) and services and instruments used in this project were supported, in part, by the University of Pittsburgh, the Office of the Senior Vice Chancellor for Health Sciences.
Author information
Authors and Affiliations
Contributions
Concept: Gitte Zels, Karen Van Baelen, Adrian Lee, Oesterreich Steffi, Giuseppe Floris and Christine Desmedt; Methodology: Gitte Zels, Karen Van Baelen, Maxim De Schepper, Anirudh Pabba, Alexander CC Chang, Adrian Lee, Oesterreich Steffi, Giuseppe Floris and Christine Desmedt; Project administration: Tatjana Geukens, Marion Maetens, Adrian Lee, Oesterreich Steffi and Christine Desmedt; Investigation: Gitte Zels, Karen Van Baelen, Anirudh Pabba, Maxim De Schepper, Raphaëla Dresen, Vincent Vandecaveye, Rohit Bhargava, Giuseppe Floris and Christine Desmedt; Resources: Gitte Zels, Karen Van Baelen, Alexander CC Chang, Anirudh Pabba, Maxim De Schepper, Marion Maetens, Josephine Van Cauwenberge, Tatjana Geukens, Kristien Borremans, François Richard, Amena Mahdami, Ha Linh Nguyen, Sophia Leduc, Patrick Neven, Hans Wildiers, Vincent Vandecaveye, Raphaëla Dresen, Wouter Van Den Bogaert, Rohit Bhargava, Tanner Bartholow, Neil Carleton, Ye Cao, Jie Bin Liu, Abdalla Wedn, Hunter Waltermire, Morgan Cody, Lori Miller, Margaret Q Rosenzweig, Julia Foldi, Marija Balic, Christoper Deibl, Christine Hodgdon, Stephanie Walker, Adrian Lee, Steffi Oesterreich, Giuseppe Floris and Christine Desmedt; Data Curation: Gitte Zels, Karen Van Baelen, Alexander CC Chang, Anirudh Pabba, Vincent Vandecaveye, Raphaëla Dresen, Adrian Lee, Steffi Oesterreich, Giuseppe Floris and Christine Desmedt; Writing Original Draft: Gitte Zels, Karen Van Baelen, Anirudh Pabba and Christine Desmedt; Writing Review & Editing: Gitte Zels, Karen Van Baelen, Alexander CC Chang, Anirudh Pabba, Maxim De Schepper, Marion Maetens, Josephine Van Cauwenberge, Tatjana Geukens, Kristien Borremans, François Richard, Amena Mahdami, Ha Linh Nguyen, Sophia Leduc, Patrick Neven, Hans Wildiers, Vincent Vandecaveye, Raphaëla Dresen, Wouter Van Den Bogaert, Rohit Bhargava, Tanner Bartholow, Neil Carleton, Ye Cao, Jie Bin Liu, Abdalla Wedn, Hunter Waltermire, Morgan Cody, Lori Miller, Margaret Q Rosenzweig, Julia Foldi, Marija Balic, Christoper Deibl, Christine Hodgdon, Stephanie Walker, Adrian Lee, Steffi Oesterreich, Giuseppe Floris and Christine Desmedt; Visualization: Gitte Zels, Karen Van Baelen, Anirudh Pabba; Supervision: Adrian Lee, Steffi Oesterreich, Giuseppe Floris and Christine Desmedt; Funding Acquisition: Gitte Zels, Karen Van Baelen, Tatjana Geukens, Marion Maetens, Adrian Lee, Steffi Oesterreich, Giuseppe Floris and Christine Desmedt
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zels, G., Van Baelen, K., Chang, A.C. et al. Clinical and histopathological characterization of metastatic lobular breast cancer: lessons learned from post-mortem tissue donation programs. npj Breast Cancer (2026). https://doi.org/10.1038/s41523-026-00912-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-026-00912-5