Abstract
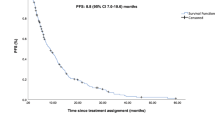
The management of HER2-negative metastatic breast cancer (MBC) in the second-line or later setting remains challenging, due to the absence of standardized regimens and the limited efficacy of chemotherapy. Here, we report a prospectively, single-arm, phase II study evaluating anlotinib plus chemotherapy in patients with HER2-negative MBC (n = 33) who had progressed after at least one prior line of systemic therapy for metastatic disease. The primary endpoints were median progression-free survival (mPFS) and overall survival (OS), while secondary endpoints included objective response rate (ORR), clinical benefit rate (CBR), disease control rate (DCR), and safety. Exploratory proteomic profiling using the Olink Target 96 Immuno-Oncology panel was performed on baseline serum samples to identify potential predictors of response. After a median follow-up of 25.9 months and the median number of prior systemic therapy lines was 2 (range, 1–4). The mPFS was 8.3 (95% CI: 6.3–10.3) months, and the mOS was 22.2(95% CI: 13.1–31.3) months. The ORR was 33.3%, DCR reached 90.9% and CBR stood at 60.6%. Proteomic analysis indicated that higher baseline serum levels of proteins including CSF-1 were associated with shorter PFS (P < 0.05). No treatment-related fatalities were observed. This trial is registered with www.chictr.org.cn (ChiCTR2400081835) on 13 March 2024.
Similar content being viewed by others
Data availability
Anonymized patient data, the research protocol, and the analysis methodology can be made available for sharing upon reasonable request and provision of a comprehensive protocol and analysis plan to the corresponding author.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J/OL]. CA: A Cancer J. Clinicians 74, 229–263 (2024).
Łukasiewicz, S. et al. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review [J/OL]. Cancers 13, 4287 (2021).
Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer[J/OL]. Ann. Oncol. 29, 1541–1547 (2018).
Finn, R. S. et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med 375, 1925–1936 (2016).
Cortes, J. et al. KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Gradishar, W. J. et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology[J/OL]. J. Natl. Compr. Cancer Netw. 22, 331–357 (2024).
Rugo, H. S. et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study[J/OL]. Lancet Oncol. 22, 489–498 (2021).
Turner, N. C. et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer [J/OL]. N. Engl. J. Med. 388, 2058–2070 (2023).
Cook, M. M. et al. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy [J/OL]. Oncologist 26, 101–106 (2021).
Bardia, A. et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer [J/OL]. N. Engl. J. Med. 384, 1529–1541 (2021).
Huppert, L. A. et al. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer[J/OL]. CA: A Cancer J. Clinicians 73, 480–515 (2023).
Pivot, X. et al. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy [J/OL]. Ann. Oncol. 27, 1525–1531 (2016).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649 (2020).
Li, C. H., Karantza, V., Aktan, G. & Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res 21, 143 (2019).
Shen, G. et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development [J/OL]. J. Hematol. Oncol. 11, 120 (2018).
Han, B. et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non–Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial[J/OL]. JAMA Oncol. 4, 1569 (2018).
Zhou, A. P. et al. Anlotinib Versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial [J/OL]. Oncologist 24, e702–e708 (2019).
Li, D. et al. Anlotinib in Locally Advanced or Metastatic Medullary Thyroid Carcinoma: A Randomized, Double-Blind Phase IIB Trial[J/OL]. Clin. Cancer Res. 27, 3567–3575 (2021).
Liu, J. et al. Phase II Study of TQB2450, a Novel PD-L1 Antibody, in Combination with Anlotinib in Patients with Locally Advanced or Metastatic Soft Tissue Sarcoma. Clin Cancer Res. 28, 3473–3479 (2022).
Qian, Y. et al. Efficacy and safety of anlotinib-based treatment in metastatic breast cancer patients[J/OL]. Front. Oncol. 12, 1042451 (2022).
Xu, T. et al. The Effects of Anlotinib Combined with Chemotherapy following Progression on Cyclin-Dependent Kinase 4/6 Inhibitor in Hormone Receptor-Positive Metastatic Breast Cancer.
LI, Y. et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2-metastatic breast cancer [J/OL]. Therapeutic Adv. Med. Oncol. 13, 175883592110228 (2021).
Cortes, J. et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study [J/OL]. Lancet 377, 914–923 (2011).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med 357, 2666–2676 (2007).
Robert, N. J. et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 29, 1252–1260 (2011).
Brufsky, A. M. et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 29, 4286–4293 (2011).
Gligorov, J. et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1351–1360 (2014).
Baselga, J. et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J. Clin. Oncol. 30, 1484–1491 (2012).
Gion, M. et al. Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial. Nat. Med 31, 2746–2754 (2025).
Mayer, E. L. et al. PACE:A randomized phase II study of fulvestrant, palbociclib, and avelumab after progression on cyclin-dependent kinase 4/6 inhibitor and aromatase inhibitor for hormone receptor-positive/human epidermal growth factor receptor-negative metastatic breast cancer [J]. J. Clin. Oncol. 42, 2050–2060 (2024).
Llombart-Cussac, A. et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR+)/human epidermal growth factor receptor2-negative (HER2-)advanced breast cancer (ABC): PALMIR Atrial [J]. J. Clin. Oncol. 41, 1001 (2023).
Kalinsky, K. et al. Randomized phase II trial of endocrine therapy with or without ribociclib after progression on cyclin-dependent kinase 4/6inhibition in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: MAINTAIN trial [j]. J. Clin. Oncol. 41, 4004–4013 (2023).
Kalinsky, K. et al. Abemaciclib plus fulvestrant vs fulvestrant alone for HR+HER2-advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: primary outcome of the phase 3 postMONARCH trial [J]. J. Clin. Oncol. 42, LBA1001 (2024).
Dhakal, A. et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib[J/OL]. Breast Cancer.: Basic Clin. Res. 14, 117822342094486 (2020).
Zhou, J. et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer[J/OL]. Breast 66, 255–261 (2022).
Modi, S. et al. DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med 387, 9–20 (2022).
Rugo, H. S. et al. Alpelisib plus fulvestrant in PIK3CAmutated, hormone receptorpositive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre,openlabel, noncomparative study[J]. Lancet Oncol. 22, 489498 (2021).
Hu, X. et al. Capivasertib plus fulvestrant in patients with HR-positive/HER2-negative advanced breast cancer: phase 3 CAPItello-291 study extended Chinese cohort. Nat. Commun. 16, 4324 (2025).
Bardia, A. et al. Abstract GS3-01:GS3-01 EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2-metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting [J]. Cancer Res 83, GS3–1 (2023).
Cussac, L. A. et al. PARSIFAL-LONG: Extended follow-up of hormone receptor-positive/HER2-negative advanced breast cancer patients treated with fulvestrant and palbociclib vs letrozole and palbociclib in the PARSIFAL study. 2023 SABCS. RF01-03.
Princic, N. et al. Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, HEGFR-2 negative metastatic breast cancer. Curr. Med Res Opin. 35, 73–80 (2019).
Carey, L. A. et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis. NPJ Breast Cancer 8, 72 (2022).
Yin, Y. et al. Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial. Nat Med. 31,1969-1975 (2025).
Lee, T. H. et al. Vascular Endothelial Growth Factor Mediates Intracrine Survival in Human Breast Carcinoma Cells through Internally Expressed VEGFR1/FLT1[J/OL]. PLoS Med. 4, e186 (2007).
Linderholm, B. K. et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer[J/OL]. Ann. Oncol. 20, 1639–1646 (2009).
Denardo, D. G. et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy [J/OL]. Cancer Discov. 1, 54–67 (2011).
Salemme, V. et al. The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity[J/OL]. Front. Oncol. 13, 1170264 (2023).
Yang, C. et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway [J/OL]. Med. Oncol. 32, 14 (2015).
Xu, X. et al. M2 macrophage-derived IL6 mediates resistance of breast cancer cells to hedgehog inhibition [J/OL]. Toxicol. Appl. Pharmacol. 364, 77–82 (2019).
Syed, Y. Y. Anlotinib: First Global Approval[J/OL]. Drugs 78, 1057–1062 (2018).
Sun, Y. et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors [J/OL]. J. Hematol. Oncol. 9, 105 (2016).
Lindgaard, S. C. et al. Circulating Protein Biomarkers for Use in Pancreatic Ductal Adenocarcinoma Identification [J/OL]. Clin. Cancer Res. 27, 2592–2603 (2021).
Acknowledgements
We gratefully acknowledge the support of all patients who participated in this study and their families, as well as the investigators and staff at each study site. This project was supported by the Beijing Xisike Clinical Oncology Research Foundation (Y-Young 2022—0072).
Author information
Authors and Affiliations
Contributions
T.X.: Collection and assembly of data, formal analysis, writing the original draft, manuscript review, and editing. Q.G.: Elisa analysis, collection, and assembly of data. S.Y.L.: formal analysis. L.L.Z.: Conceptualization, data curation, project administration, supervision; Y.Y.: Conceptualization, data curation, project administration, supervision, and writing–review and editing. Y.Y. and L.L.Z. are considered co-corresponding authors, and Y.Y. is the predominant corresponding author. All authors were responsible for the decision to submit the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, T., Gu, Q., Li, S. et al. Phase II trial of anlotinib-chemotherapy combination in pretreated HER2-negative metastatic breast cancer: therapeutic efficacy and proteomic biomarker profiling. npj Breast Cancer (2026). https://doi.org/10.1038/s41523-026-00914-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-026-00914-3