Abstract
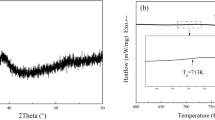
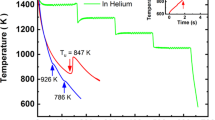
Understanding thermophysical properties such as surface tension (σ), total hemispherical emissivity (ε), specific heat capacity (cp) and viscosity (η) as a function of temperature is essential for optimizing the vitrification of bulk metallic glasses (BMGs). In this study, the thermophysical properties of liquid Vit106a were measured aboard the International Space Station (ISS) using the electromagnetic levitator (EML). The surface tension σ exhibited a similar value with other Zr-based BMG, with a weak temperature dependence described by σ(T) = 1.557–4.36 ×10−5 × (T - 1106) N.m−1. The viscosity temperature-dependence η(T) was analyzed using the Vogel–Fulcher–Tammann (VFT) equation, yielding a kinetic fragility parameter of D* = 9.8 at high temperature, compared to D* = 21.6 at low temperature, that indicates a fragile-to-strong transition characteristic of Zr-based metallic glass formers. XRD analysis confirms full crystallization of the sample, despite being cooled at a rate of 16 K.s⁻¹, over nine times faster than the critical cooling rate of 1.75 K.s⁻¹ reported in the literature. The crystallized sample reveals a heterogeneous distribution of binary intermetallic phases, including ZrAl3, Zr2Cu, Zr2Ni, ZrAl and Nb2Ni. These findings provide insights into the thermophysical behavior of liquid Vit106a for large-scale manufacturing but also raise important questions regarding its good glass-forming ability for larger casting thickness.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Wang, W. H., Dong, C. & Shek, C. H. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 44, 45–89 (2004).
Miller, M. K., Liaw, P. K. (eds) Bulk Metallic Glasses: An Overview (Springer, 2008).
Peker, A. & Johnson, W. L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10.0Be22.5. Appl. Phys. Lett. 63, 2342–2344 (1993).
Löffler, J. F. & Johnson, W. L. Model for decomposition and crystallization of Zr-based bulk amorphous alloys near the glass transition. Mater. Sci. Eng. A 304–306, 670–673 (2001).
Wei, S. et al. Linking structure to fragility in bulk metallic glass-forming liquids. Appl. Phys. Lett. 106, 181901 (2015).
Mohr, M., Wunderlich, R. K., Hofmann, D. C. & Fecht, H.-J. Thermophysical properties of liquid Zr52.5Cu17.9Ni14.6Al10Ti5—prospects for bulk metallic glass manufacturing in space. npj Microgravity 5, 24 (2019).
Gangopadhyay, A. K. et al. Demonstration of the effect of stirring on nucleation from experiments on the international space station using the ISS-EML facility. npj Microgravity 7, 31 (2021).
Jurewicz, A. J. G. et al. The Genesis solar-wind collector materials. In The Genesis Mission (ed. Russell, C. T.) (Springer Netherlands, 2003; pp 27–52.
Hays, C. C. et al. Vitrification and determination of the crystallization time scales of the bulk-metallic-glass-forming liquid Zr58.5Nb2.8Cu15.6Ni12.8Al10.3. Appl. Phys. Lett. 79, 1605–1607 (2001).
Stolpe, M. et al. Structural changes during a liquid-liquid transition in the deeply undercooled Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 bulk metallic glass forming melt. Phys. Rev. B 93, 014201 (2016).
Bendert, J. C., Blodgett, M. E., Gangopadhyay, A. K. & Kelton, K. F. Measurements of volume, thermal expansion, and specific heat in Zr57Cu15.4Ni12.6Al10Nb5 and Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 liquids and glasses. Appl. Phys. Lett. 102, 211913 (2013).
Haag, F. et al. Assessing continuous casting of precious bulk metallic glasses. J. Non Crystalline Solids 521, 119120 (2019).
Pei, Z. & Ju, D. Simulation of the continuous casting and cooling behavior of metallic glasses. Materials 10, 420 (2017).
Tang, R., Zhou, B., Ma, Y., Jia, F. & Zhang, X. Numerical simulation of Zr-based bulk metallic glass during continuous casting solidification process. Mat. Res. 18, 3–9 (2015).
Yang, E., Ding, T. & Ren, T. The temperature field characteristics and amorphous formation ability during the continuous casting process of Zr-based bulk metallic glass. Heliyon 10, e37626 (2024).
Fecht, H.-J., Mohr, M. (eds) Metallurgy in Space: Recent Results from ISS; The Minerals, Metals & Materials Series (Springer International Publishing, 2022). https://doi.org/10.1007/978-3-030-89784-0.
Mohr, M. et al. Surface tension and viscosity of liquid Pd43Cu27Ni10P20 measured in a levitation device under microgravity. npj Microgravity 5, 4 (2019).
Mohr, M., Hofmann, D. C. & Fecht, H.-J. Thermophysical properties of an Fe57.75Ni19.25Mo10C5B8 glass-forming alloy measured in microgravity. Adv. Eng. Mater. 23, 2001143 (2021).
Mohr, M. et al. Electromagnetic levitation containerless processing of metallic materials in microgravity: thermophysical properties. npj Microgravity 9, 34 (2023).
Mohr, M. et al. Surface tension and viscosity of Cu50Zr50 measured by the oscillating drop technique on board the international space station. Microgravity Sci. Technol. 31, 177–184 (2019).
Blodgett, M. E., Egami, T., Nussinov, Z. & Kelton, K. F. Proposal for universality in the viscosity of metallic liquids. Sci. Rep. 5, 13837 (2015).
Kelton, K. F. A perspective on metallic liquids and glasses. J. Appl. Phys. 134, https://doi.org/10.1063/5.0144250 (2003).
Hofmann, D. C. & Roberts, S. N. Microgravity metal processing: from undercooled liquids to bulk metallic glasses. npj Microgravity 1, 15003 (2015).
Fan, C., Choo, H. & Liaw, P. K. Influences of Ta, Nb, or Mo additions in Zr-based bulk metallic glasses on microstructure and thermal properties. Scr. Mater. 53, 1407–1410 (2005).
Rayleigh, L. On the capillary phenomena of jets. Proc. R. Soc. 29, 71–97 (1879).
Lamb, H. Hydrodynamics, 450 (Cambridge University Press, Cambridge, 1975).
Fecht, H. J. & Johnson, W. L. A conceptual approach for noncontact calorimetry in space. Rev. Sci. Instrum. 62, 1299–1303 (1991).
Wunderlich, R. K., Fecht, H.-J. & Willnecker, R. Power modulation technique for noncontact high-temperature calorimetry. Appl. Phys. Lett. 62, 3111–3113 (1993).
Evenson, Z., Schmitt, T., Nicola, M., Gallino, I. & Busch, R. High temperature melt viscosity and fragile to strong transition in Zr–Cu–Ni–Al–Nb(Ti) and Cu47Ti34Zr11Ni8 bulk metallic glasses. Acta Mater. 60, 4712–4719 (2012).
Evenson, Z., Raedersdorf, S., Gallino, I. & Busch, R. Equilibrium viscosity of Zr–Cu–Ni–Al–Nb bulk metallic glasses. Scr. Mater. 63, 573–576 (2010).
Gallino, I. On the fragility of bulk metallic glass forming liquids. Entropy 19, 483 (2017).
Mukherjee, S., Schroers, J., Zhou, Z., Johnson, W. L. & Rhim, W.-K. Viscosity and specific volume of bulk metallic glass-forming alloys and their correlation with glass forming ability. Acta Mater. 52, 3689–3695 (2004).
Gallino, I., Shah, M. B. & Busch, R. Enthalpy relaxation and its relation to the thermodynamics and crystallization of the Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 bulk metallic glass-forming alloy. Acta Mater. 55, 1367–1376 (2007).
Evenson, Z., Gallino, I. & Busch, R. The effect of cooling rates on the apparent fragility of Zr-based bulk metallic glasses. J. Appl. Phys. 107, 123529 (2010).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Busch, R. The thermophysical properties of bulk metallic glass-forming liquids. JOM 52, 39–42 (2000).
Shadowspeaker, L., Shah, M. & Busch, R. On the crystalline equilibrium phases of the Zr57Cu15.4Ni12.6Al10Nb5 bulk metallic glass forming alloy. Scr. Mater. 50, 1035–1038 (2004).
Wilke, S. K. et al. Microgravity effects on nonequilibrium melt processing of neodymium titanate: thermophysical properties, atomic structure, glass formation and crystallization. npj Microgravity 10, 26 (2024).
Krasovskyy, V. P., Naidich, Y. V. & Krasovskaya, N. A. Surface tension and density of copper–zirconium alloys in contact with fluoride refractories. J. Mater. Sci. 40, 2367–2369 (2005).
Li, X. et al. Surface tension and viscosity of Zr–Ti–Cu liquid alloys. Vacuum 220, 112712 (2024).
Mehta, U. et al. Study of surface tension and viscosity of Cu–Fe–Si ternary alloy using a thermodynamic approach. Heliyon 6, e04674 (2020).
Wei, S. et al. Liquid–liquid transition in a strong bulk metallic glass-forming liquid. Nat. Commun. 4, 2083 (2013).
Way, C., Wadhwa, P. & Busch, R. The influence of shear rate and temperature on the viscosity and fragility of the Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 metallic-glass-forming liquid. Acta Mater. 55, 2977–2983 (2007).
Li, J. J. Z., Rhim, W. K., Kim, C. P., Samwer, K. & Johnson, W. L. Evidence for a liquid–liquid phase transition in metallic fluids observed by electrostatic levitation. Acta Mater.a 59, 2166–2171 (2011).
Mukherjee, S., Zhou, Z., Schroers, J., Johnson, W. L. & Rhim, W. K. Overheating threshold and its effect on time–temperature-transformation diagrams of zirconium based bulk metallic glasses. Appl. Phys. Lett. 84, 5010–5012 (2004).
Murty, B. S., Ping, D. H., Hono, K. & Inoue, A. Influence of oxygen on the crystallization behavior of Zr₆₅Cu₂₇.₅Al₇.₅ and Zr₆₆.₇Cu₃₃.₃ metallic glasses. Acta Mater. 48, 3985–3996 (2000).
Lin, X. H., Johnson, W. L. & Rhim, W. K. Effect of oxygen impurity on crystallization of an undercooled bulk glass forming Zr–Ti–Cu–Ni–Al alloy. Mater. Trans. JIM 38, 473–477 (1997).
Sosso, G. C. et al. Crystal nucleation in liquids: open questions and future challenges in molecular dynamics simulations. Chem. Rev. 116, 7078–7116 (2016).
Zhang, P., Maldonis, J. J., Liu, Z., Schroers, J. & Voyles, P. M. Spatially heterogeneous dynamics in a metallic glass forming liquid imaged by electron correlation microscopy. Nat. Commun. 9, 1129 (2018).
Zanotto, E. D. & Montazerian, M. Dominant effect of heterogeneous dynamics on homogenous crystal nucleation in supercooled liquids. Front. Phys. 8, 20 (2020).
Yang, G. N., Shao, Y., Yao, K. F. & Chen, S. Q. A study of cooling process in bulk metallic glasses fabrication. AIP Adv. 5, 117111 (2015).
Acknowledgements
Funding sources: The authors are grateful for funding from the ThermoProp-CH project, related to the European Space Agency MAP AO-99-022 and the PRODEX Experiment Arrangement No 4000115323 (PI: AN).
Author information
Authors and Affiliations
Contributions
D.T. wrote the main manuscript text. H.-J.F., A.D. and A.N. conceived the project and funding. R.W. and A.N. designed the experiments. S.S. conducted the experiments via remote control of the EML from Earth. M.M. and R.W. analyzed the thermophysical properties. D.T., M.M., S.S. and R.W. contributed to the interpretation of the thermophysical results. D.T. performed the microstructural characterization using SEM and XRD. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terebenec, D., Mohr, M., Wunderlich, R. et al. Thermophysical properties and solidification behavior of liquid Vit106a in microgravity. npj Microgravity (2026). https://doi.org/10.1038/s41526-026-00572-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-026-00572-6