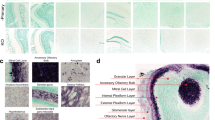
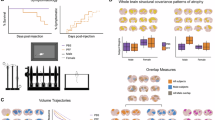
Abstract
In Lewy body disease, alpha-synucleinopathic inclusions tend to develop in brain regions that extend long, thin, and unmyelinated projection fibers, but it is not known if this selective vulnerability is caused by poor myelination status. Our objective was to investigate this link in the limbic forebrain. First, we observed that levels of insoluble, hyperphosphorylated alpha-synuclein correlated inversely with the myelin marker proteolipid protein (20 kDa isoform) in the amygdala of men (but not women) with Lewy body disease. Second, select oligodendrocytic markers were mildly suppressed in the amygdala of men with Lewy body disease. Third, preformed fibrils (PFFs) of alpha-synuclein tended to induce greater pathology in gray matter, even when infused into murine brain regions penetrated by myelinated fiber bundles. Given these correlative patterns, we tested the hypothesis that myelin disruption with pharmacologic or genetic tools will exacerbate the development of limbic alpha-synucleinopathy in mice of both sexes, using a full-factorial experimental design. In nontransgenic mice with retrobulbar infusions of PFFs, dietary exposure to the established myelin disruptor cuprizone modestly increased the fraction of Triton-insoluble alpha-synuclein that was hyperphosphorylated at serine 129 (pSer129). Contrary to our hypothesis, however, cuprizone did not induce systematic, robust worsening of most pSer129+ object attributes, behavior deficits, cellular toxicity measures (NeuN+/Hoechst+ object attributes), or pan-alpha-synuclein insolubility. Similarly, PFF-induced effects on the insolubility and hyperphosphorylation of alpha-synuclein, behavior measures, and pSer129+, NeuN+, and Hoechst+ object attributes were not markedly amplified in mice heterozygous for the shiverer mutation in myelin basic protein (Mbp+/shi), compared to wild-type littermates. Although alpha-synucleinopathy is negatively associated with select myelin markers in mice and men, the causal nature of this link thus remains to be verified, and its potential implications are discussed here.

Similar content being viewed by others
Data availability
Full datasets for the bulk RNA-seq analyses are available at NCBI’s Gene Expression Omnibus (ID number GSE297212). The underlying code for this study is available on GitHub and can be accessed via this link: https://github.com/RachelClarkDUQ/RC_Cuprizone-Shiverer-Paper?tab=readme-ov-file#rc_cuprizone-shiverer-paper Other data will be made available upon request. Please email leakr@duq.edu.
References
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R. & Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426 (2002).
Braak, H. et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J. Neurol. 249, 1–5 (2002).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Braak, H., Rub, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Angot, E., Steiner, J. A., Hansen, C., Li, J. Y. & Brundin, P. Are synucleinopathies prion-like disorders? Lancet Neurol. 9, 1128–1138 (2010).
Burre, J., Sharma, M. & Sudhof, T. C. Cell Biology And Pathophysiology Of Alpha-synuclein. Cold Spring Harb. Perspect. Med. 8, https://doi.org/10.1101/cshperspect.a024091 (2018).
Chu, Y. & Kordower, J. H. The prion hypothesis of Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 15, 28 (2015).
Leak, R. K., Frosch, M. P., Beach, T. G. & Halliday, G. M. Alpha-synuclein: prion or prion-like? Acta Neuropathol. 138, 509–514 (2019).
Tamguney, G. & Korczyn, A. D. A critical review of the prion hypothesis of human synucleinopathies. Cell Tissue Res. 373, 213–220 (2018).
Volpicelli-Daley, L. & Brundin, P. Prion-like propagation of pathology in Parkinson's disease. Handb. Clin. Neurol. 153, 321–335 (2018).
Rey, N. L., Wesson, D. W. & Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. https://doi.org/10.1016/j.nbd.2016.12.013 (2016).
Braak, H., Ghebremedhin, E., Rub, U., Bratzke, H. & Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318, 121–134 (2004).
Orimo, S. et al. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 37, 791–802 (2011).
Stadelmann, C., Timmler, S., Barrantes-Freer, A. & Simons, M. Myelin in the central nervous system: structure, function, and pathology. Physiol. Rev. 99, 1381–1431 (2019).
Hattori, T. et al. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum. Brain Mapp. 33, 727–739 (2012).
Auning, E. et al. White matter integrity and cognition in Parkinson’s disease: a cross-sectional study. BMJ Open 4, e003976 (2014).
Braak, H. et al. Parkinson’s disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol. 99, 489–495 (2000).
Braak, H. & Del Tredici, K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol. Aging 25, 19–23 (2004).
Hatton, C. et al. Prion-like alpha-synuclein pathology in the brain of infants with Krabbe disease. Brain 145, 1257–1263 (2022).
Agarwal, D. et al. A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat. Commun. 11, 4183 (2020).
Bryois, J. et al. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat. Genet 52, 482–493 (2020).
Reynolds, R. H. et al. Moving beyond neurons: the role of cell type-specific gene regulation in Parkinson’s disease heritability. NPJ Parkinsons Dis. 5, 6 (2019).
Smajic, S. et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain, https://doi.org/10.1093/brain/awab446 (2021).
Adams, L., Song, M. K., Yuen, S., Tanaka, Y. & Kim, Y. S. A single-nuclei paired multiomic analysis of the human midbrain reveals age- and Parkinson’s disease-associated glial changes. Nat. Aging 4, 364–378 (2024).
Bae, E. J., Perez-Acuna, D., Rhee, K. H. & Lee, S. J. Changes in oligodendroglial subpopulations in Parkinson’s disease. Mol. Brain 16, 65 (2023).
Martirosyan, A. et al. Unravelling cell type-specific responses to Parkinson’s Disease at single cell resolution. Mol. Neurodegener. 19, 7 (2024).
Gallagher, C. et al. White matter microstructural integrity and executive function in Parkinson’s disease. J. Int. Neuropsychol. Soc. 19, 349–354 (2013).
Camacho, M. et al. Exploiting macro- and micro-structural brain changes for improved Parkinson’s disease classification from MRI data. NPJ Parkinsons Dis. 10, 43 (2024).
Bercury, K. K. & Macklin, W. B. Dynamics and mechanisms of CNS myelination. Dev. Cell 32, 447–458 (2015).
min, Y. et al. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc. Natl. Acad. Sci. USA 106, 3154–3159 (2009).
Jahn, O. et al. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front. Cell Neurosci. 14, 239 (2020).
Ruskamo, S. et al. Human myelin proteolipid protein structure and lipid bilayer stacking. Cell Mol. Life Sci. 79, 419 (2022).
Miner, K. M. et al. The variance in phosphorylated, insoluble α-synuclein in humans, rats, and mice is not mainly driven by biological sex. Acta Neuropathol. 146, 651–654 (2023).
Bhatia, T. N. et al. Heat Shock Protein 70 as a Sex-Skewed Regulator of alpha-Synucleinopathy. Neurotherapeutics 18, 2541–2564 (2021).
Miner, K. M. et al. alpha-synucleinopathy exerts sex-dimorphic effects on the multipurpose DNA repair/redox protein APE1 in mice and humans. Prog. Neurobiol. 216, 102307 (2022).
Harauz, G. & Boggs, J. M. Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J. Neurochem 125, 334–361 (2013).
Akiyama, K., Ichinose, S., Omori, A., Sakurai, Y. & Asou, H. Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J. Neurosci. Res. 68, 19–28 (2002).
Boggs, J. M. Myelin basic protein: a multifunctional protein. Cell Mol. Life Sci. 63, 1945–1961 (2006).
Rasband, M. N. & Macklin, W. B. in Basic Neurochemistry (Eighth Edition) (eds Scott T. Brady, George J. Siegel, R. Wayne Albers, & Donald L. Price) 180-199 (Academic Press, 2012).
Harauz, G. et al. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron 35, 503–542 (2004).
Johns, T. G. & Bernard, C. C. The structure and function of myelin oligodendrocyte glycoprotein. J. Neurochem. 72, 1–9 (1999).
Zhuo, Z. et al. Brain structural and functional alterations in MOG antibody disease. Mult. Scler. 27, 1350–1363 (2021).
Solly, S. K. et al. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia 18, 39–48 (1996).
Paumier, K. L. et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 82, 185–199 (2015).
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Bullock, D. N. et al. A taxonomy of the brain’s white matter: twenty-one major tracts for the 21st century. Cereb. Cortex 32, 4524–4548 (2022).
Sacino, A. N. et al. Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 127, 645–665 (2014).
Uemura, N. et al. Slow Progressive Accumulation of Oligodendroglial Alpha-Synuclein (alpha-Syn) Pathology in Synthetic alpha-Syn Fibril-Induced Mouse Models of Synucleinopathy. J. Neuropathol. Exp. Neurol. 78, 877–890 (2019).
Nouraei, N. et al. Critical appraisal of pathology transmission in the alpha-synuclein fibril model of Lewy body disorders. Exp. Neurol. 299, 172–196 (2018).
Leak, R. K. & Moore, R. Y. Identification of retinal ganglion cells projecting to the lateral hypothalamic area of the rat. Brain Res. 770, 105–114 (1997).
Leak, R. K. et al. Current insights and assumptions on alpha-synuclein in Lewy body disease. Acta Neuropathol. 148, 18 (2024).
Mason, J. L. et al. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J. Neurosci. Res. 61, 251–262 (2000).
Mason, J. L. et al. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am. J. Pathol. 164, 1673–1682 (2004).
Skripuletz, T., Gudi, V., Hackstette, D. & Stangel, M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol. Histopathol. 26, 1585–1597 (2011).
Kipp, M. How to Use the Cuprizone Model to Study De- and Remyelination. Int. J. Mol. Sci. 25, https://doi.org/10.3390/ijms25031445 (2024).
Matsushima, G. K. & Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 11, 107–116 (2001).
Lindner, M., Fokuhl, J., Linsmeier, F., Trebst, C. & Stangel, M. Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci. Lett. 453, 120–125 (2009).
Franco-Pons, N., Torrente, M., Colomina, M. T. & Vilella, E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol. Lett. 169, 205–213 (2007).
Liu, L. et al. Myelin repair is accelerated by inactivating CXCR2 on nonhematopoietic cells. J. Neurosci. 30, 9074–9083 (2010).
Sachs, H. H., Bercury, K. K., Popescu, D. C., Narayanan, S. P. & Macklin, W. B. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro 6, 1759091414551955 (2014).
Steelman, A. J., Thompson, J. P. & Li, J. Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci. Res. 72, 32–42 (2012).
Stidworthy, M. F., Genoud, S., Suter, U., Mantei, N. & Franklin, R. J. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 13, 329–339 (2003).
Skurnack, A. M. E. et al. Voluntary Wheel Running an Effective Intervention in the Management of Excessive Food Usage in CD-1 Mice (Mus musculus). J. Am. Assoc. Lab Anim. Sci. 63, 504–512 (2024).
Zhan, J. et al. The Cuprizone Model: dos and do nots. Cells 9, https://doi.org/10.3390/cells9040843 (2020).
McMurran, C. E., Zhao, C. & Franklin, R. J. M. Toxin-Based Models to Investigate Demyelination and Remyelination. Methods Mol. Biol. 1936, 377–396 (2019).
Goldberg, J., Clarner, T., Beyer, C. & Kipp, M. Anatomical Distribution of Cuprizone-Induced Lesions in C57BL6 Mice. J. Mol. Neurosci. 57, 166–175 (2015).
Hubner, N. S. et al. The connectomics of brain demyelination: Functional and structural patterns in the cuprizone mouse model. NeuroImage 146, 1–18 (2017).
Silvestroff, L. et al. Cuprizone-induced demyelination in CNP::GFP transgenic mice. J. Comp. Neurol. 518, 2261–2283 (2010).
Vega-Riquer, J. M., Mendez-Victoriano, G., Morales-Luckie, R. A. & Gonzalez-Perez, O. Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases. Curr. Neuropharmacol. 17, 129–141 (2019).
Yang, H. J. et al. Region-specific susceptibilities to cuprizone-induced lesions in the mouse forebrain: Implications for the pathophysiology of schizophrenia. Brain Res. 1270, 121–130 (2009).
Yu, Q. et al. Strain differences in cuprizone induced demyelination. Cell Biosci. 7, 59 (2017).
Hiremath, M. M. et al. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol. 92, 38–49 (1998).
Rey, N. L. et al. Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med. https://doi.org/10.1084/jem.20160368 (2016).
Brunjes, P. C., Kay, R. B. & Arrivillaga, J. P. The mouse olfactory peduncle. J. Comp. Neurol. 519, 2870–2886 (2011).
Bagchi, B. et al. Disruption of myelin leads to ectopic expression of K(V)1.1 channels with abnormal conductivity of optic nerve axons in a cuprizone-induced model of demyelination. PLoS One 9, e87736 (2014).
Ikeda, M. & Oka, Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2, 382–390 (2012).
Bhatia, T. N. et al. A 14-day pulse of PLX5622 modifies alpha-synucleinopathy in preformed fibril-infused aged mice of both sexes. Neurobiol Dis, 106196, https://doi.org/10.1016/j.nbd.2023.106196 (2023).
Silveira-Moriyama, L. et al. Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci. Lett. 453, 77–80 (2009).
Adler, C. H. & Beach, T. G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 31, 1114–1119 (2016).
Mason, D. M. et al. The center of olfactory bulb-seeded alpha-synucleinopathy is the limbic system and the ensuing pathology is higher in male than in female mice. Brain Pathol. 29, 741–770 (2019).
Parra-Rivas, L. A. et al. Serine-129 phosphorylation of alpha-synuclein is an activity-dependent trigger for physiologic protein-protein interactions and synaptic function. Neuron 111, 4006–4023.e4010 (2023).
Ramalingam, N. et al. Dynamic physiological alpha-synuclein S129 phosphorylation is driven by neuronal activity. NPJ Parkinsons Dis. 9, 4 (2023).
Killinger, B. A. et al. Distribution of phosphorylated alpha-synuclein in non-diseased brain implicates olfactory bulb mitral cells in synucleinopathy pathogenesis. NPJ Parkinsons Dis. 9, 43 (2023).
Harding, A. J., Stimson, E., Henderson, J. M. & Halliday, G. M. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125, 2431–2445 (2002).
Hsieh, L. S., Wen, J. H., Miyares, L., Lombroso, P. J. & Bordey, A. Outbred CD1 mice are as suitable as inbred C57BL/6J mice in performing social tasks. Neurosci. Lett. 637, 142–147 (2017).
Dues, D. J., Nguyen, A. P. T., Becker, K., Ma, J. & Moore, D. J. Hippocampal subfield vulnerability to alpha-synuclein pathology precedes neurodegeneration and cognitive dysfunction. NPJ Parkinsons Dis. 9, 125 (2023).
Dean, D. C. et al. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS One 11, e0163774 (2016).
Yang, K. et al. White matter changes in Parkinson’s disease. NPJ Parkinsons Dis. 9, 150 (2023).
Ibarretxe-Bilbao, N. et al. Olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas: A voxel-based diffusion tensor imaging study. Mov. Disord. 25, 1888–1894 (2010).
Chiang, P. L. et al. White matter damage and systemic inflammation in Parkinson’s disease. BMC Neurosci. 18, 48 (2017).
Zhang, K. et al. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur. J. Radio. 77, 269–273 (2011).
Joki, H. et al. White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J. Neurol. Sci. 385, 99–104 (2018).
Bozzali, M. et al. Brain tissue damage in dementia with Lewy bodies: an in vivo diffusion tensor MRI study. Brain 128, 1595–1604 (2005).
Nedelska, Z. et al. White matter integrity in dementia with Lewy bodies: a voxel-based analysis of diffusion tensor imaging. Neurobiol. Aging 36, 2010–2017 (2015).
Maass, F. et al. Myelin basic protein and TREM2 quantification in the CSF of patients with Multiple System Atrophy and other Parkinsonian conditions. J. Neurol. 272, 52 (2024).
Wang, Q. et al. Molecular profiling of human substantia nigra identifies diverse neuron types associated with vulnerability in Parkinson’s disease. Sci. Adv. 10, eadi8287 (2024).
Salazar Campos, J. M., Burbulla, L. F. & Jakel, S. Are oligodendrocytes bystanders or drivers of Parkinson’s disease pathology? PLoS Biol. 23, e3002977 (2025).
Fitzgerald, J. C. et al. Interactions of Oligodendrocyte Precursor Cells and Dopaminergic Neurons in the Mouse Substantia Nigra. J. Neurochem 169, e16298 (2025).
Grace, A. A. & Bunney, B. S. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science 210, 654–656 (1980).
Geertsma, H. M. et al. A topographical atlas of alpha-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs. NPJ Parkinsons Dis. 10, 65 (2024).
Asi, Y. T. et al. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia 62, 964–970 (2014).
Reyes, J. F. et al. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia 62, 387–398 (2014).
Wenning, G. K., Stefanova, N., Jellinger, K. A., Poewe, W. & Schlossmacher, M. G. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 64, 239–246 (2008).
Kon, T. et al. SNCA and TPPP transcripts increase in oligodendroglial cytoplasmic inclusions in multiple system atrophy. Neurobiol. Dis. 198, 106551 (2024).
Rieker, C. et al. Neuropathology in mice expressing mouse alpha-synuclein. PLoS One 6, e24834 (2011).
Giasson, B. I. et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34, 521–533 (2002).
Ferreira, N. et al. Trans-synaptic spreading of alpha-synuclein pathology through sensory afferents leads to sensory nerve degeneration and neuropathic pain. Acta Neuropathol. Commun. 9, 31 (2021).
van der Putten, H. et al. Neuropathology in mice expressing human alpha-synuclein. J. Neurosci. 20, 6021–6029 (2000).
Arai, T. et al. Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/alpha-synuclein. Neurosci. Lett. 259, 83–86 (1999).
Stevenson, T. J. et al. alpha-synuclein inclusions are abundant in non-neuronal cells in the anterior olfactory nucleus of the Parkinson’s disease olfactory bulb. Sci. Rep. 10, 6682 (2020).
Brunjes, P. C., Illig, K. R. & Meyer, E. A. A field guide to the anterior olfactory nucleus (cortex). Brain Res. Brain Res Rev. 50, 305–335 (2005).
Collins, L. N., Hill, D. L. & Brunjes, P. C. Myelination of the developing lateral olfactory tract and anterior commissure. J. Comp. Neurol. 526, 1843–1858 (2018).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s The mouse brain in stereotaxic coordinates. Fourth edition. edn, (Academic Press, an imprint of Elsevier, 2013).
Beach, T. G. et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 117, 169–174 (2009).
Ubeda-Banon, I. et al. alpha-Synucleinopathy in the human olfactory system in Parkinson’s disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol. 119, 723–735 (2010).
Pearce, R. K., Hawkes, C. H. & Daniel, S. E. The anterior olfactory nucleus in Parkinson’s disease. Mov. Disord. 10, 283–287 (1995).
Mason, D. M. et al. Transmission of alpha-synucleinopathy from olfactory structures deep into the temporal lobe. Mol. Neurodegener. 11, 49 (2016).
Mattson, M. P. & Leak, R. K. The hormesis principle of neuroplasticity and neuroprotection. Cell Metab. 36, 315–337 (2024).
Kimura, M. et al. Restoration of myelin formation by a single type of myelin basic protein in transgenic shiverer mice. Proc. Natl. Acad. Sci. USA 86, 5661–5665 (1989).
Chernoff, G. F. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J. Hered. 72, 128 (1981).
Readhead, C. & Hood, L. The dysmyelinating mouse mutations shiverer (shi) and myelin-deficient (shimld). Behav. Genet. 20, 213–234 (1990).
Poggi, G. et al. Cortical network dysfunction caused by a subtle defect of myelination. Glia 64, 2025–2040 (2016).
Barbarese, E., Nielson, M. L. & Carson, J. H. The effect of the shiverer mutation on myelin basic protein expression in homozygous and heterozygous mouse brain. J. Neurochem. 40, 1680–1686 (1983).
Martin, M. et al. Myelin deficiencies visualized in vivo: visually evoked potentials and T2-weighted magnetic resonance images of shiverer mutant and wild-type mice. J. Neurosci. Res. 84, 1716–1726 (2006).
Cammer, W., Kahn, S. & Zimmerman, T. Biochemical abnormalities in spinal cord myelin and CNS homogenates in heterozygotes affected by the shiverer mutation. J. Neurochem. 42, 1372–1378 (1984).
Taylor, L. C., Puranam, K., Gilmore, W., Ting, J. P. & Matsushima, G. K. 17beta-estradiol protects male mice from cuprizone-induced demyelination and oligodendrocyte loss. Neurobiol. Dis. 39, 127–137 (2010).
Taylor, L. C., Gilmore, W., Ting, J. P. & Matsushima, G. K. Cuprizone induces similar demyelination in male and female C57BL/6 mice and results in disruption of the estrous cycle. J. Neurosci. Res. 88, 391–402 (2010).
Van Loo, P. L. P. et al. Strain-specific aggressive behavior of male mice submitted to different husbandry procedures. Aggress. Behav. 29, 69–80 (2003).
Cum, M. et al. A Multiparadigm Approach to Characterize Dominance Behaviors in CD1 and C57BL6 Male Mice. eNeuro 11, https://doi.org/10.1523/ENEURO.0342-24.2024 (2024).
Lidster, K., Owen, K., Browne, W. J. & Prescott, M. J. Cage aggression in group-housed laboratory male mice: an international data crowdsourcing project. Sci. Rep. 9, 15211 (2019).
Polinski, N. K. et al. Best practices for generating and using Alpha-synuclein pre-formed fibrils to Model Parkinson’s disease in rodents. J. Parkinsons Dis. 8, 303–322 (2018).
Volpicelli-Daley, L. A., Luk, K. C. & Lee, V. M. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014).
Arbuckle, E. P., Smith, G. D., Gomez, M. C. & Lugo, J. N. Testing for odor discrimination and habituation in mice. JoVE (J. Vis. Exp.), e52615 (2015).
Dellu, F., Contarino, A., Simon, H., Koob, G. & Gold, L. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol. Learn. Mem. 73, 31–48 (2000).
Gross, M. & Pinhasov, A. Chronic mild stress in submissive mice: Marked polydipsia and social avoidance without hedonic deficit in the sucrose preference test. Behav. Brain Res. 298, 25–34 (2016).
Watson, R. E. Jr., Wiegand, S. J., Clough, R. W. & Hoffman, G. E. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7, 155–159 (1986).
Dorph-Petersen, K. A. & Lewis, D. A. Stereological approaches to identifying neuropathology in psychosis. Biol. Psychiatry 69, 113–126 (2011).
Petersen, M. S., Petersen, C. C., Agger, R., Hokland, M. & Gundersen, H. J. A simple method for unbiased quantitation of adoptively transferred cells in solid tissues. J. Immunol. Methods 309, 173–181 (2006).
Gundersen, H. J., Jensen, E. B., Kieu, K. & Nielsen, J. The efficiency of systematic sampling in stereology-reconsidered. J. Microsc 193, 199–211 (1999).
Beirowski, B. et al. Metabolic regulator LKB1 is crucial for Schwann cell–mediated axon maintenance. Nat. Neurosci. 17, 1351–1361 (2014).
Cognat, E., Cleophax, S., Domenga-Denier, V. & Joutel, A. Early white matter changes in CADASIL: evidence of segmental intramyelinic oedema in a pre-clinical mouse model. Acta Neuropathol. Commun. 2, 49 (2014).
Beirowski, B., Wong, K. M., Babetto, E. & Milbrandt, J. mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells. Proc. Natl. Acad. Sci. 114, E4261–E4270 (2017).
Goebbels, S. et al. Elevated phosphatidylinositol 3, 4, 5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 30, 8953–8964 (2010).
Sharon, R. et al. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. USA 98, 9110–9115 (2001).
Acknowledgements
We are grateful for support by the NIH (1R15NS130532-01 and 1R21NS141002-01 to RKL). MA was supported by a Fulbright Scholarship from the Institute of International Education. We thank Walter Clark for outstanding assistance with the RStudio script. We are grateful to Drs. Johnnie Anderson and Torbjørn Kristensen at VisioPharm for their support with stereological measurements. We thank the Animal Care Facility (Rachel Barr, Ashley Yuhouse, Peyton Jackson, and Lindsay Hudson), the Center for Biologic Imaging at the University of Pittsburgh, the Graduate School of Pharmaceutical Sciences, and the Duquesne University Office of Research and Innovation for their kind support. We are grateful to Dr. Brian Popko for shipping us the shiverer breeders. JC is the Richard King Mellon Professor of Neurology at the University of Pittsburgh and supported by the VA Senior Research Career Scientist Award (821-RC-NB-30556) and Merit Review awards (I01BX005290 and I01BX003377) from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
R.N.C. contributed to the methodology, software, validation, formal analysis, investigation, resources, visualization, and writing the original draft as well as review and editing. R.E.L. contributed to the methodology, formal analysis, investigation, writing the original draft as well as reviewing and editing the manuscript. M.A. and J.R.J contributed to methodology, investigation, and formal analysis. V.G.M., K.A.D., and J.F. contributed to formal analysis and investigation. J.C., K.C.L., and X.H. contributed to reviewing and editing the manuscript. R.K.L. contributed to the conceptualization, methodology, validation, formal analysis, investigation, resources, writing the original draft as well as review and editing, visualization, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Clark, R.N., Landes, R.E., Abbas, M. et al. Testing an inverse link between limbic alpha-synucleinopathy and myelin markers in mice and humans. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-026-01278-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-026-01278-y