Abstract
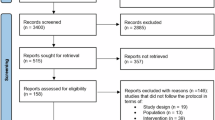
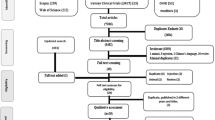
Pressurised metered dose inhalers (pMDIs) contain propellant gases with high global warming potential yet remain a cornerstone of management for asthma and chronic obstructive pulmonary disease (COPD). The aim of this study was to determine whether non-propellant alternatives of dry powder inhalers (DPIs) and soft mist inhalers (SMIs) had similar efficacy and safety. A systematic review was performed finding 44 randomised trials (24,710 participants) and moderate certainty evidence for most outcomes. No statistically significant or clinically important differences were found between inhaler types for any assessed measure. For asthma maintenance, the mean difference in peak expiratory flow rate between groups was 1.07 L/min (95% confidence interval [CI] -0.93 to 3.06). For COPD, the mean difference in FEV1 between groups was 0.01 L (95% CI -0.01 to 0.02). While the choice of optimal inhaler for an individual patient is a multifaceted decision, this review provides reassurance that non-pMDI devices can perform equally well.
Similar content being viewed by others
Data availability
Our research protocol is publicly available on the Monash University research repository (https://doi.org/10.26180/26065789.v2). The datasets generated and/or analysed during the current study are also available on the Monash University research repository (https://doi.org/10.26180/28916777.v2).
References
Safiri, S. et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. Bmj 378, e069679 (2022).
Wang, Z. et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir. Res. 24, 169 (2023).
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, www.ginasthma.org (2023).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD, www.goldcopd.org (2023).
MCTOC: Medical and Chemical Technical Options Committee. 2018 Assessment Report, https://ozone.unep.org/sites/default/files/2019-04/MCTOC-Assessment-Report-2018.pdf (2018).
Wilkinson, A. J. K., Braggins, R., Steinbach, I. & Smith, J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ open. 9, e028763 (2019).
Lenzen, M. et al. The environmental footprint of health care: a global assessment. Lancet Planet. Health 4, e271–e279 (2020).
Pichler, P.-P., Jaccard, I. S., Weisz, U. & Weisz, H. International comparison of health care carbon footprints. Environ. Res. Lett. 14, 064004 (2019).
NHS England. Delivering a Net Zero National Health Service, https://www.england.nhs.uk/greenernhs/a-net-zero-nhs/ (2021).
Lavorini, F. et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir. Med. 105, 1099–1103 (2011).
Wilkinson, A. & Woodcock, A. The environmental impact of inhalers for asthma: A green challenge and a golden opportunity. Br. J. Clin. Pharmacol. 88, 3016–3022 (2022).
British Thoracic Society and Scottish Intercollegiate Guideline Network. BTS/SIGN British guideline on the management of asthma: A national clinical guideline, https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (2019).
Brocklebank, D., Wright, J. & Cates, C. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering corticosteroids in asthma. Bmj 323, 896–900 (2001).
Dolovich, M. B. et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest 127, 335–371 (2005).
Ram, F. S., Brocklebank, D. M., Muers, M., Wright, J. & Jones, P. W. Pressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2002, Cd002170 (2002).
Ram, F. S., Wright, J., Brocklebank, D. & White, J. E. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering beta (2)agonists bronchodilators in asthma. Bmj 323, 901–905 (2001).
Department of Health and Aged Care. National Health and Climate Strategy, https://www.health.gov.au/our-work/national-health-and-climate-strategy (2023).
Loftus M. J. et al. Systematic Review Protocol - Metered Dose inhalers versus Dry Powder Inhalers and Soft Mist Inhalers for Asthma and COPD, https://doi.org/10.26180/26065789.v2 (2024).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
National Institute for Health and Care Excellence (NICE). Inhaled corticosteroid doses for NICE’s asthma guideline, https://www.nice.org.uk/guidance/ng80/resources/inhaled-corticosteroid-doses-pdf-4731528781 (2023).
Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia, 2024).
Rohatgi A. WebPlotDigitizer version 5.1, https://automeris.io (2024).
Higgins J. P. T. & Altman D. G. in Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins J. P. T. & Green S.) (John Wiley & Sons, 2008).
The Cochrane Collaboration. Review Manager (RevMan). Version 8.5.2, www.revman.cochrane.org (2024).
Schünemann HJ, H. J. et al. in Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (ed Thomas J. Higgins J. P. T., Chandler J., Cumpston M., Li T., Page M. J., Welch V. A.) (Cochrane, 2023).
Santesso, N. et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 119, 126–135 (2020).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur. Respir. J. 26, 948–968 (2005).
Donohue, J. F. Minimal clinically important differences in COPD lung function. Copd 2, 111–124 (2005).
Santanello, N. C., Zhang, J., Seidenberg, B., Reiss, T. F. & Barber, B. L. What are minimal important changes for asthma measures in a clinical trial?. Eur. Respir. J. 14, 23–27 (1999).
Karras, D. J. et al. Clinically meaningful changes in quantitative measures of asthma severity. Acad. Emerg. Med. 7, 327–334 (2000).
Dissanayake, S., Jain, M., Grothe, B., McIver, T. & Papi, A. An evaluation of comparative treatment effects with high and low dose fluticasone propionate/formoterol combination in asthma. Pulmonary Pharmacology Therapeutics 35, 19–27 (2015).
Costello, A. et al. Managing the health effects of climate change: lancet and university college london institute for global health commission. Lancet 373, 1693–1733 (2009).
Vicedo-Cabrera, A. M. et al. Climate change and respiratory health: a european respiratory society position statement. Eur. Respir. J. 62, 2201960 (2023).
Pernigotti, D. et al. Reducing carbon footprint of inhalers: analysis of climate and clinical implications of different scenarios in five European countries. BMJ Open. Respir. Res. 8, e001071 (2021).
Murphy, A., Howlett, D., Gowson, A. & Lewis, H. Understanding the feasibility and environmental effectiveness of a pilot postal inhaler recovery and recycling scheme. NPJ Prim. Care Respir. Med. 33, 5 (2023).
Wilkinson, A. J. K. et al. Greenhouse gas emissions associated with suboptimal asthma care in the UK: the SABINA healthCARe-Based envirONmental cost of treatment (CARBON) study. Thorax 79, 412–421 (2024).
Lavorini, F., Janson, C., Braido, F., Stratelis, G. & Løkke, A. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther. Adv. Respir. Dis. 13, 1753466619884532 (2019).
British Thoracic Society. Position Statement Sustainability and the Environment: Climate Change & Lung Health, https://www.brit-thoracic.org.uk/about-us/position-statements/ (2024).
National Institute for Health and Care Excellence (NICE). Patient Decision Aid: Inhalers for Asthma, https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573/ (2020).
Parker, J. Barriers to green inhaler prescribing: ethical issues in environmentally sustainable clinical practice. J. Med. Ethics 49, 92–98 (2023).
Keeley, D., Scullion, J. E. & Usmani, O. S. Minimising the environmental impact of inhaled therapies: problems with policy on low carbon inhalers. Eur. Respir. J. 55, 2001122 (2020).
D’Ancona, G., Cumella, A., Renwick, C. & Walker, S. The sustainability agenda and inhaled therapy: what do patients want?. European Respiratory Journal 58, PA3399 (2021).
Liew, K. & Wilkinson, A. P280 How do we choose inhalers? patient and physician perspectives on environmental, financial and ease-of-use factors. Thorax 72, A235–A237 (2017).
Metting, E. I., Dijk, L. V., Messlaki, H. E., Luers, J. & Kock, J. Development of a shared decision-making tool to support patients and their healthcare provider in choosing the best inhaler device. Eur. Respiratory J. 52, OA1643 (2018).
Woodcock, A. et al. The environmental impact of inhaled therapy: making informed treatment choices. Eur. Respir. J. 60, 2102106 (2022).
Clark, A. R., Weers, J. G. & Dhand, R. The confusing world of dry powder inhalers: it is all about inspiratory pressures, not inspiratory flow rates. J. Aerosol Med. Pulm. Drug. Deliv. 33, 1–11 (2020).
Mahler, D. A., Waterman, L. A., Ward, J. & Gifford, A. H. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J. Aerosol Med. Pulm. Drug. Deliv. 27, 103–109 (2014).
Sharma, G. et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr. Pulm. Dis. 4, 217–224 (2017).
Kuek, S. L. et al. Dry powder inhaler use in primary school aged children with asthma: a systematic review. ERJ Open. Res. 10, 00455–02024 (2024).
Price, D. B. et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J. Allergy Clin. Immunol. Pract. 5, 1071–1081.e1079 (2017).
Usmani, O. S. et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir. Res. 19, 10 (2018).
Vestbo, J. et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N. Engl. J. Med. 375, 1253–1260 (2016).
Woodcock, A. et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 390, 2247–2255 (2017).
Jones, R. et al. The comparative effectiveness of initiating fluticasone/salmeterol combination therapy via pMDI versus DPI in reducing exacerbations and treatment escalation in COPD: a UK database study. Int. J. Chron. Obstruct Pulmon Dis. 12, 2445–2454 (2017).
Price, D. et al. Device type and real-world effectiveness of asthma combination therapy: an observational study. Respir. Med. 105, 1457–1466 (2011).
Usmani, O. S. et al. Real-world impact of nonclinical inhaler regimen switches on asthma or COPD: a systematic review. J. Allergy Clin. Immunol. Pract. 10, 2624–2637 (2022).
Thomas, M. et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm. Med. 9, 1 (2009).
Price, D. et al. Switching patients from other inhaled corticosteroid devices to the Easyhaler(®): historical, matched-cohort study of real-life asthma patients. J. Asthma Allergy 7, 31–51 (2014).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline: Choice of Control Group and Related Issues in Clinical Trials E10. ICH: Geneva, 2000.
Amar, N. J., Shekar, T., Varnell, T. A., Mehta, A. & Philip, G. Mometasone furoate (MF) improves lung function in pediatric asthma: A double-blind, randomized controlled dose-ranging trial of MF metered-dose inhaler. Pediatr. Pulmonol. 52, 310–318 (2017).
Corrigendum. Pediatr Pulmonol. 54: 655–656. https://doi.org/10.1002/ppul.24291.
Barnes, N. et al. Stepping-across controlled asthmatic patients to extrafine beclometasone/formoterol combination. Pulm. Pharmacol. Ther. 26, 555–561 (2013).
Bateman, E. D., Silins, V. & Bogolubov, M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 microg twice daily) when administered via a chlorofluorocarbon-free metered dose inhaler or dry powder inhaler to patients with mild-to-moderate asthma. Respir. Med. 95, 136–146 (2001).
Bernstein, D. I. et al. Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma. Allergy Asthma Clin. Immunol. 7, 21 (2011).
Bodzenta-Lukaszyk, A. et al. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J. Asthma 49, 1060–1070 (2012).
Bracamonte, T., Schauer, U., Emeryk, A., Godwood, A. & Balsara, S. Efficacy and safety of salmeterol/fluticasone propionate combination delivered by the diskustrade mark or pressurised metered-dose inhaler in children with asthma. Clin. Drug. Investig. 25, 1–11 (2005).
Bronsky, E. et al. Comparison of inhaled albuterol powder and aerosol in asthma. J. Allergy Clin. Immunol. 79, 741–747 (1987).
Busse, W. W. et al. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. J. Allergy Clin. Immunol. 121, 1407–1414 (2008). 1414.e1401-1406.
O’Connor, R. D., Patrick, D. L., Parasuraman, B., Martin, P. & Goldman, M. Comparison of patient-reported outcomes during treatment with adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler versus fixed-dose fluticasone propionate/salmeterol dry powder inhaler in patients with asthma. J. Asthma 47, 217–223 (2010).
Dusser, D., Vicaut, E. & Lefrançois, G. Double-blind, double-dummy, multinational, multicenter, parallel-group design clinical trial of clinical non-inferiority of formoterol 12 microg/unit dose in a b.i.d. regimen administered via an HFA-propellant-pMDI or a dry powder inhaler in a 12-week treatment period of moderate to severe stable persistent asthma in adult patients. Respiration 72, 20–27 (2005).
Kanniess, F., Scuri, M., Vezzoli, S., Francisco, C. & Petruzzelli, S. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler(®)) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: A randomised, double-blind trial. Pulm. Pharmacol. Ther. 30, 121–127 (2015).
Kemp, J. P. et al. Albuterol treatment for children with asthma: a comparison of inhaled powder and aerosol. J. Allergy Clin. Immunol. 83, 697–702 (1989).
Koskela, T. et al. Equivalence of two steroid-containing inhalers: easyhaler multidose powder inhaler compared with conventional aerosol with large-volume spacer. Respiration 67, 194–202 (2000).
Lundback, B. et al. Evaluation of fluticasone propionate (500 micrograms day-1) administered either as dry powder via a Diskhaler inhaler or pressurized inhaler and compared with beclomethasone dipropionate (1000 micrograms day-1) administered by pressurized inhaler. Respir. Med. 87, 609–620 (1993).
Lundback, B. et al. A comparison of fluticasone propionate when delivered by either the metered-dose inhaler or the Diskhaler inhaler in the treatment of mild-to-moderate asthma. Eur. J. Clin. Res. 5, 11–19 (1994).
Morice, A. H., Peterson, S., Beckman, O. & Osmanliev, D. Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma. Int. J. Clin. Pract. 61, 1874–1883 (2007).
Morice, A. H., Hochmuth, L., Ekelund, J., Thorén, A. & Puterman, A. S. Comparable long-term safety and efficacy of a novel budesonide/formoterol pressurized metered-dose inhaler versus budesonide/formoterol Turbuhaler in adolescents and adults with asthma. Pulm. Pharmacol. Ther. 21, 32–39 (2008).
Nelson, H., Kemp, J. P., Bieler, S., Vaughan, L. M. & Hill, M. R. Comparative efficacy and safety of albuterol sulfate Spiros inhaler and albuterol metered-dose inhaler in asthma. Chest 115, 329–335 (1999).
Papi, A., Paggiaro, P. L., Nicolini, G., Vignola, A. M. & Fabbri, L. M. Beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur. Respir. J. 29, 682–689 (2007).
Papi, A. et al. Step-down from high dose fixed combination therapy in asthma patients: a randomized controlled trial. Respir. Res. 13, 54 (2012).
Pauwels, R. A., Hargreave, F. E., Camus, P., Bukoski, M. & Ståhl, E. A 1-year comparison of turbuhaler vs pressurized metered-dose inhaler in asthmatic patients. Chest 110, 53–57 (1996).
Poukkula, A. et al. Comparison of a multidose powder inhaler containing beclomethasone dipropionate (BDP) with a BDP metered dose inhaler with spacer in the treatment of asthmatic patients. Clin. Drug. Investig. 16, 101–110 (1998).
Reichel, W. et al. Extrafine beclomethasone dipropionate breath-actuated inhaler (400 micrograms/day) versus budesonide dry powder inhaler (800 micrograms/day) in asthma. Int. J. Clin. Pract. 55, 100–106 (2001).
Srichana, T., Juthong, S., Thawithong, E., Supaiboonpipat, S. & Soorapan, S. Clinical equivalence of budesonide dry powder inhaler and pressurized metered dose inhaler. Clin. Respir. J. 10, 74–82 (2016).
Stradling, J. R., Pearson, M. G., Morice, A. H., Peake, M. D. & Barnes, N. C. Efficacy and safety of a novel beclomethasone dipropionate dry powder inhaler (Clickhaler) for the treatment of adult asthma. Amsterdam Clinical Study Group. J. Asthma 37, 183–190 (2000).
van Noord, J. A., Lill, H., Diaz, T. C., Greefhorst, A. P. & Davies, P. Clinical equivalence of a salmeterol/fluticasone propionate combination product (50/500μg) delivered via a chlorofluorocarbon-free metered-dose inhaler with the diskus™ in patients with moderate to severe asthma. Clin. Drug. Investigation 21, 243–255 (2001).
Vincken, W., Bantje, T., Middle, M. V., Gerken, F. & Moonen, D. Long-term efficacy and safety of ipratropium bromide plus fenoterol via respimat((R)) soft misttrade mark inhaler (SMI) versus a pressurised metered-dose inhaler in asthma. Clin. Drug. Investig. 24, 17–28 (2004).
von Berg, A. et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via respimat soft mist inhaler vs. a conventional metered dose inhaler plus spacer in children with asthma. Pediatr. Pulmonol. 37, 264–272 (2004).
von Berg, A. et al. Comparison of the efficacy and safety of ciclesonide 160 microg once daily vs. budesonide 400 microg once daily in children with asthma. Pediatr. Allergy Immunol. 18, 391–400 (2007).
Wardlaw, A. et al. Efficacy and safety of mometasone furoate dry powder inhaler vs fluticasone propionate metered-dose inhaler in asthma subjects previously using fluticasone propionate. Ann. Allergy Asthma Immunol. 93, 49–55 (2004).
Wolfe, J. et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Ann. Allergy Asthma Immunol. 84, 334–340 (2000).
Zheng, J. et al. Comparison of extrafine beclomethasone dipropionate/formoterol fumarate dry powder inhaler and pressurized metered-dose inhaler in Chinese patients with asthma: the FORTUNE study. J. Asthma 61, 360–367 (2024).
Zhou, Y. et al. Efficacy of inhaler devices in new-onset mild asthma: a randomized blinded multicenter trial. Adv. Ther. 42, 3861–3881 (2025).
Direkwatanachai, C. et al. Comparison of salbutamol efficacy in children-via the metered-dose inhaler (MDI) with Volumatic spacer and via the dry powder inhaler, Easyhaler, with the nebulizer-in mild to moderate asthma exacerbation: a multicenter, randomized study. Asian Pac. J. Allergy Immunol. 29, 25–33 (2011).
Drblik, S. et al. Comparative efficacy of terbutaline sulphate delivered by Turbuhaler dry powder inhaler or pressurised metered dose inhaler with Nebuhaler spacer in children during an acute asthmatic episode. Arch. Dis. Child. 88, 319–323 (2003).
Khaled, M. S., Akter, F., Rahman, K., Ullah, M. S. & Rahman, M. H. Bronchodilator response to salbutamol delivered by metered dose inhaler with spacer and dry powder inhaler in acute asthma in children: a comparative study. Bangladesh J. Child. Health 38, 68–73 (2014).
Lodha, R., Gupta, G., Baruah, B. P., Nagpal, R. & Kabra, S. K. Metered dose inhaler with spacer versus dry powder inhaler for delivery of salbutamol in acute exacerbations of asthma: a randomized controlled trial. Indian. Pediatr. 41, 15–20 (2004).
Vangveeravong, M. A comparative study of efficacy of salbutamol via metered dose inhaler with volumatic spacer and via dry powder inhaler, easyhaler, to nebulization in mild to moderate severity acute asthma exacerbation in childhood. J. Med. Assoc. Thai 91(Suppl 3), S115–S123 (2008).
Ferguson, G. T., Ghafouri, M., Dai, L. & Dunn, L. J. COPD patient satisfaction with ipratropium bromide/albuterol delivered via Respimat: a randomized, controlled study. Int. J. Chron. Obstruct Pulmon Dis. 8, 139–150 (2013).
Ferguson, G. T. et al. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur. Respir. J. 52, 1801334 (2018).
Kilfeather, S. A. et al. Improved delivery of ipratropium bromide/fenoterol from Respimat Soft Mist Inhaler in patients with COPD. Respir. Med. 98, 387–397 (2004).
Koser, A., Westerman, J., Sharma, S., Emmett, A. & Crater, G. D. Safety and efficacy of fluticasone propionate/salmeterol hydrofluoroalkane 134a metered-dose-inhaler compared with fluticasone propionate/salmeterol diskus in patients with chronic obstructive pulmonary disease. Open. Respir. Med. J. 4, 86–91 (2010).
Maltais, F. et al. A randomized, double-blind, double-dummy study of glycopyrrolate/formoterol fumarate metered dose inhaler relative to umeclidinium/vilanterol dry powder inhaler in COPD. Adv. Ther. 36, 2434–2449 (2019).
Wang, C. et al. Efficacy and safety of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in chinese patients with COPD: a subgroup analysis of KRONOS. Adv. Ther. 37, 1591–1607 (2020).
Zuwallack, R. et al. Efficacy and safety of ipratropium bromide/albuterol delivered via respimat inhaler versus MDI. Respir. Med. 104, 1179–1188 (2010).
Acknowledgements
Thanks to Dr Kim Jachno of the Australian Living Evidence Collaboration in the School of Public Health and Preventive Medicine, Monash University, for statistical advice. Michael Loftus is supported by a Royal Australasian College of Physicians (RACP) Research Establishment Fellowship. Miranda Cumpston is supported by philanthropic research funding from the Walter Thomas Cottman Charitable Trust and The Phyllis Connor Memorial Trust, managed by Equity Trustees. The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
Author Contribution Statement : MJL: conceptualization, methodology, investigation, formal analysis, writing – original draft, writing – review and editing. MSC: methodology, investigation, visualisation, formal analysis, writing – original draft, writing – review and editing. SB: investigation, writing – review and editing. JB: conceptualization, methodology, supervision, writing – original draft, writing – original draft, writing – review and editing. AG: conceptualization, methodology, supervision, writing – original draft, writing – original draft, writing – review and editing. SM: methodology, investigation, writing – review and editing. LP: investigation, writing – review and editing. MR: conceptualization, methodology, supervision, writing – original draft, writing – original draft, writing – review and editing. RS: investigation, writing – review and editing. HW: methodology, investigation, writing – review and editing. TT: conceptualization, methodology, supervision, writing – review and editing. KL: conceptualization, methodology, supervision, writing – original draft, writing – original draft, writing – review and editing. All authors (except AG, who died in December 2024) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Author JB declares personal and institutional fees from Chiesi, Boehringer Ingelheim, GSK, AstraZeneca and Sanofi for educational presentations or speaking engagements, as well as support for travelling to conferences from GSK and AstraZeneca. Author JB has participated in a study steering committee for GSK, unrelated to this topic. All other authors declare no financial or non-financial competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loftus, M.J., Cumpston, M.S., Barnes, S. et al. Efficacy and safety of different inhaler types for asthma and chronic obstructive pulmonary disease. a systematic review and meta-analysis. npj Prim. Care Respir. Med. (2026). https://doi.org/10.1038/s41533-026-00488-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-026-00488-4