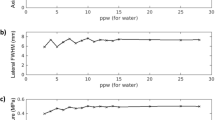
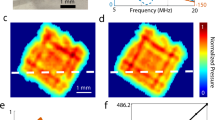
Abstract
Optical and ultrasonic techniques for imaging the living brain have traditionally been limited to low-resolution interrogations or highly invasive craniotomy procedures. Localization-based techniques for super-resolution ultrasound and optical imaging, as well as hybrid optoacoustic techniques, are now enabling multiscale interrogations of the brain to exploit anatomical, functional and molecular contrasts non-invasively or minimally invasively. However, the skull bone remains a substantial obstacle to the transcranial application of light- and sound-based imaging techniques. Our knowledge of the skull’s acoustic properties inherited from transcranial ultrasound has been primarily limited to a narrowband and normal-incidence-angle detection regimen, which is inapplicable to more advanced ultrasound and optoacoustic brain imaging technology. In this Perspective, we examine the transcranial wave-propagation problem, as well as recent efforts to characterize and model skull-induced distortions and develop compensatory strategies. We then summarize recent preclinical and human applications of brain imaging and delve into the most pressing challenges facing this dynamic field at the crossroads of physics, engineering and medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sjöstedt, E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, eaay5947 (2020).
Moussavi, S. et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858 (2007).
Lépine, J.-P. & Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 7, 3–7 (2011).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Kalender, W. A. X-ray computed tomography. Phys. Med. Biol. 51, R29–R43 (2006).
Rahmim, A. & Zaidi, H. PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun. 29, 193–207 (2008).
Eggebrecht, A. T. et al. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photonics 8, 448–454 (2014).
Demené, C. et al. Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients. Nat. Biomed. Eng. 5, 219–228 (2021).
Yao, J. & Wang, L. V. Photoacoustic brain imaging: from microscopic to macroscopic scales. Neurophotonics 1, 011003 (2014).
Fry, F. J. & Barger, J. E. Acoustical properties of the human skull. J. Acoust. Soc. Am. 63, 1576–1590 (1978).
Tanter, M., Thomas, J.-L. & Fink, M. Focusing and steering through absorbing and aberrating layers: application to ultrasonic propagation through the skull. J. Acoust. Soc. Am. 103, 2403–2410 (1998).
Clement, G. T. & Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 47, 1219–1236 (2002).
Aubry, J.-F., Tanter, M., Pernot, M., Thomas, J.-L. & Fink, M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J. Acoust. Soc. Am. 113, 84–93 (2003).
Kyriakou, A. et al. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int. J. Hyperthermia 30, 36–46 (2014).
Estrada, H., Rebling, J. & Razansky, D. Prediction and near-field observation of skull-guided acoustic waves. Phys. Med. Biol. 62, 4728–4740 (2017).
Estrada, H. et al. Observation of guided acoustic waves in a human skull. Ultrasound Med. Biol. 44, 2388–2392 (2018).
Mazzotti, M., Kohtanen, E., Erturk, A. & Ruzzene, M. Radiation characteristics of cranial leaky lamb waves. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 68, 2129–2140 (2021).
Mazzotti, M., Kohtanen, E., Erturk, A. & Ruzzene, M. Optimizing transcranial ultrasound delivery at large incident angles by leveraging cranial leaky guided wave dispersion. Ultrasonics 128, 106882 (2023).
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Na, S. et al. Massively parallel functional photoacoustic computed tomography of the human brain. Nat. Biomed. Eng. 6, 584–592 (2022).
Zhang, Y. et al. Transcranial photoacoustic computed tomography of human brain function. Preprint at https://doi.org/10.48550/arXiv.2206.00248 (2022).
Ni, R. et al. Coregistered transcranial optoacoustic and magnetic resonance angiography of the human brain. Opt. Lett. 48, 648–651 (2023).
Tiran, E. et al. Transcranial functional ultrasound imaging in freely moving awake mice and anesthetized young rats without contrast agent. Ultrasound Med. Biol. 43, 1679–1689 (2017).
Imbault, M., Chauvet, D., Gennisson, J.-L., Capelle, L. & Tanter, M. Intraoperative functional ultrasound imaging of human brain activity. Sci. Rep. 7, 7304 (2017).
Demene, C. et al. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 9, eaah6756 (2017).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Chen, Z., Zhou, Q., Robin, J. & Razansky, D. Widefield fluorescence localization microscopy for transcranial imaging of cortical perfusion with capillary resolution. Opt. Lett. 45, 3470–3473 (2020).
Zhou, Q. et al. Diffuse optical localization imaging for noninvasive deep brain microangiography in the NIR-II window. Optica 8, 796–803 (2021).
Dean-Ben, X. L. & Razansky, D. Localization optoacoustic tomography. Light Sci. Appl. 7, 18004 (2018).
Zhang, P., Li, L., Lin, L., Shi, J. & Wang, L. V. In vivo superresolution photoacoustic computed tomography by localization of single dyed droplets. Light Sci. Appl. 8, 36 (2019).
Deán-Ben, X. L. et al. Deep optoacoustic localization microangiography of ischemic stroke in mice. Nat. Commun. 14, 3584 (2023).
Kim, J. et al. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 8, 103 (2019).
Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photon 8, 723–730 (2014).
Qian, Y. et al. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat. Methods 16, 171–174 (2019).
Shemetov, A. A. et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat. Biotechnol. 39, 368–377 (2021).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Møllgård, K. et al. A mesothelium divides the subarachnoid space into functional compartments. Science 379, 84–88 (2023).
Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37–R61 (2013).
Brookes, M. & Revell, W. J. in Blood Supply of Bone: Scientific Aspects 64–74 (Springer, 1998)
Waters, J. Sources of widefield fluorescence from the brain. eLife 9, e59841 (2020).
Bashkatov, A. N., Genina, E. A., Kochubey, V. I. & Tuchin, V. V. Optical properties of human cranial bone in the spectral range from 800 to 2000 nm. In Proc. SPIE Vol. 6163, 616310 (SPIE, 2006).
Li, W. et al. Tracking strain-specific morphogenesis and angiogenesis of murine calvaria with large-scale optoacoustic and ultrasound microscopy. J. Bone Miner. Res. 37, 1032–1043 (2022).
Yücel, M. A., Selb, J. J., Huppert, T. J., Franceschini, M. A. & Boas, D. A. Functional near infrared spectroscopy: enabling routine functional brain imaging. Curr. Opin. Biomed. Eng. 4, 78–86 (2017).
Chen, W.-L. et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front. Neurosci. 14, 724 (2020).
Culver, J. P., Ntziachristos, V., Holboke, M. J. & Yodh, A. G. Optimization of optode arrangements for diffuse optical tomography: a singular-value analysis. Opt. Lett. 26, 701–703 (2001).
Rayleigh, L. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 17, 4–11 (1885).
Lamb, H. On waves in an elastic plate. Proc. R. Soc. A 93, 114–128 (1917).
Jing, B., Strassle Rojas, S. & Lindsey, B. D. Effect of skull porosity on ultrasound transmission and wave mode conversion at large incidence angles. Med. Phys. 50, 3092–3102 (2023).
Webb, T. D. et al. Acoustic attenuation: multifrequency measurement and relationship to CT and MR imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 68, 1532–1545 (2021).
Estrada, H., Rebling, J., Turner, J. & Razansky, D. Broadband acoustic properties of a murine skull. Phys. Med. Biol. 61, 1932–1946 (2016).
Pinton, G. et al. Attenuation, scattering, and absorption of ultrasound in the skull bone. Med. Phys. 39, 299–307 (2012).
Estrada, H. et al. Virtual craniotomy for high-resolution optoacoustic brain microscopy. Sci. Rep. 8, 1459 (2018).
Elias, W. J. et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 375, 730–739 (2016).
Aubry, J.-F., Tanter, M., Thomas, J.-L. & Fink, M. in Acoustical Imaging (eds Halliwell, M. & Wells, P. N. T.) 101–108 (Springer, 2000).
Clement, G. T., White, P. J. & Hynynen, K. Enhanced ultrasound transmission through the human skull using shear mode conversion. J. Acoust. Soc. Am. 115, 1356–1364 (2004).
Sammartino, F., Beam, D. W., Snell, J. & Krishna, V. Kranion, an open-source environment for planning transcranial focused ultrasound surgery: technical note. J. Neurosurg. 132, 1249–1255 (2019).
Pajek, D. & Hynynen, K. The design of a focused ultrasound transducer array for the treatment of stroke: a simulation study. Phys. Med. Biol. 57, 4951–4968 (2012).
Leung, S. A., Webb, T. D., Bitton, R. R., Ghanouni, P. & Butts Pauly, K. A rapid beam simulation framework for transcranial focused ultrasound. Sci. Rep. 9, 7965 (2019).
Schoen, S. & Arvanitis, C. D. Heterogeneous angular spectrum method for trans-skull imaging and focusing. IEEE Trans. Med. Imaging 39, 1605–1614 (2020).
Yoon, K., Lee, W., Croce, P., Cammalleri, A. & Yoo, S.-S. Multi-resolution simulation of focused ultrasound propagation through ovine skull from a single-element transducer. Phys. Med. Biol. 63, 105001 (2018).
Clement, G. T. & Hynynen, K. Correlation of ultrasound phase with physical skull properties. Ultrasound Med. Biol. 28, 617–624 (2002).
Aubry, J.-F. et al. Benchmark problems for transcranial ultrasound simulation: intercomparison of compressional wave models. J. Acoust. Soc. Am. 152, 1003–1019 (2022).
Pichardo, S. et al. A viscoelastic model for the prediction of transcranial ultrasound propagation: application for the estimation of shear acoustic properties in the human skull. Phys. Med. Biol. 62, 6938–6962 (2017).
Treeby, B. E. & Cox, B. T. Modeling power law absorption and dispersion for acoustic propagation using the fractional Laplacian. J. Acoust. Soc. Am. 127, 2741–2748 (2010).
Webb, T. D. et al. Measurements of the relationship between CT Hounsfield units and acoustic velocity and how it changes with photon energy and reconstruction method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 1111–1124 (2018).
McCann, C. M. et al. Combined magnetic resonance and fluorescence imaging of the living mouse brain reveals glioma response to chemotherapy. NeuroImage 45, 360–369 (2009).
Wang, T. et al. Three-photon imaging of mouse brain structure and function through the intact skull. Nat. Methods 15, 789–792 (2018).
Kalchenko, V., Israeli, D., Kuznetsov, Y. & Harmelin, A. Transcranial optical vascular imaging (TOVI) of cortical hemodynamics in mouse brain. Sci. Rep. 4, 5839 (2014).
Chen, C. et al. High-resolution two-photon transcranial imaging of brain using direct wavefront sensing. Photon Res. 9, 1144–1156 (2021).
Heo, C. et al. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 6, 27818 (2016).
Mestre, H. et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367, eaax7171 (2020).
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373, eabf9277 (2021).
Zhao, Y.-J. et al. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light Sci. Appl. 7, 17153 (2018).
Kalchenko, V. et al. A robust method for adjustment of laser speckle contrast imaging during transcranial mouse brain visualization. Photonics 6, 80 (2019).
Barson, D. et al. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat. Methods 17, 107–113 (2020).
Chen, Z. et al. High-speed large-field multifocal illumination fluorescence microscopy. Laser Photonics Rev. 14, 1900070 (2020).
Ni, R. et al. Detection of cerebral tauopathy in P301L mice using high-resolution large-field multifocal illumination fluorescence microscopy. Biomed. Opt. Express 11, 4989–5002 (2020).
Xu, J., Song, S., Wei, W. & Wang, R. K. Wide field and highly sensitive angiography based on optical coherence tomography with akinetic swept source. Biomed. Opt. Express 8, 420–435 (2017).
Mu, J. et al. The chemistry of organic contrast agents in the NIR-II window. Angew. Chem. Int. Ed. 61, e202114722 (2022).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Sieu, L.-A. et al. EEG and functional ultrasound imaging in mobile rats. Nat. Methods 12, 831–834 (2015).
Lebas, H. et al. Imaging cerebral arteries tortuosity and velocities by transcranial Doppler ultrasound is a reliable assessment of brain aneurysm in mouse models. Stroke Vasc. Interv. Neurol. 3, e000476 (2023).
Bertolo, A. et al. Whole-brain 3D activation and functional connectivity mapping in mice using transcranial functional ultrasound imaging. J. Vis. Exp. 168, e62267 (2021).
Rabut, C. et al. 4D functional ultrasound imaging of whole-brain activity in rodents. Nat. Methods 16, 994–997 (2019).
Chavignon, A. et al. D transcranial ultrasound localization microscopy in the rat brain with a multiplexed matrix probe. IEEE Trans. Biomed. Eng. 69, 2132–2142 (2022).
Weber, J., Beard, P. C. & Bohndiek, S. E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 13, 639–650 (2016).
Gottschalk, S. et al. Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain. Nat. Biomed. Eng. 3, 392–401 (2019).
Mc Larney, B., Hutter, M. A., Degtyaruk, O., Dean-Ben, X. L. & Razansky, D. Monitoring of stimulus evoked murine somatosensory cortex hemodynamic activity with volumetric multi-spectral optoacoustic tomography. Front. Neurosci. 14, 536 (2020).
Razansky, D., Klohs, J. & Ni, R. Multi-scale optoacoustic molecular imaging of brain diseases. Eur. J. Nucl. Med. Mol. Imaging 48, 4152–4170 (2021).
Liu, X. et al. Targeted photoacoustic imaging of brain tumor mediated by neutrophils engineered with lipid-based molecular probe. ACS Mater. Lett. 3, 1284–1290 (2021).
Ni, R., Vaas, M., Ren, W. & Klohs, J. Noninvasive detection of acute cerebral hypoxia and subsequent matrix-metalloproteinase activity in a mouse model of cerebral ischemia using multispectral-optoacoustic-tomography. Neurophotonics 5, 015005 (2018).
Wang, B., Xiao, J. & Jiang, H. Simultaneous real-time 3D photoacoustic tomography and EEG for neurovascular coupling study in an animal model of epilepsy. J. Neural Eng. 11, 046013 (2014).
Yang, X., Chen, Y.-H., Xia, F. & Sawan, M. Photoacoustic imaging for monitoring of stroke diseases: a review. Photoacoustics 23, 100287 (2021).
Estrada, H. et al. High-resolution fluorescence-guided transcranial ultrasound mapping in the live mouse brain. Sci. Adv. 7, eabi5464 (2021).
Maslov, K., Zhang, H. F., Hu, S. & Wang, L. V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 33, 929–931 (2008).
Ning, B. et al. Ultrasound-aided multi-parametric photoacoustic microscopy of the mouse brain. Sci. Rep. 5, 18775 (2015).
Yao, J. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 12, 407–410 (2015).
Obrig, H. NIRS in clinical neurology—a ‘promising’ tool? NeuroImage 85, 535–546 (2014).
Lee, C. W., Cooper, R. J. & Austin, T. Diffuse optical tomography to investigate the newborn brain. Pediatr. Res. 82, 376–386 (2017).
Huo, C. et al. A review on functional near-infrared spectroscopy and application in stroke rehabilitation. Med. Nov. Technol. Devices 11, 100064 (2021).
Kirsch, J. D., Mathur, M., Johnson, M. H., Gowthaman, G. & Scoutt, L. M. Advances in transcranial Doppler US: imaging ahead. Radiographics 33, E1–E14 (2013).
Marinoni, M., Ginanneschi, A., Forleo, P. & Amaducci, L. Technical limits in transcranial Doppler recording: inadquate acoustic windows. Ultrasound Med. Biol. 23, 1275–1277 (1997).
Bercoff, J. et al. Ultrafast compound Doppler imaging: providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 134–147 (2011).
Baranger, J. et al. Bedside functional monitoring of the dynamic brain connectivity in human neonates. Nat. Commun. 12, 1080 (2021).
Zhou, S. et al. Transcranial volumetric imaging using a conformal ultrasound patch. Nature 629, 810–818 (2024).
Rabut, C. et al. Functional ultrasound imaging of human brain activity through an acoustically transparent cranial window. Sci. Transl. Med. 16, eadj3143 (2024).
Deán-Ben, X. L., Gottschalk, S., Mc Larney, B., Shoham, S. & Razansky, D. Advanced optoacoustic methods for multiscale imaging of in vivo dynamics. Chem. Soc. Rev. 46, 2158–2198 (2017).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Demené, C. et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans. Med. Imaging 34, 2271–2285 (2015).
Baranger, J. et al. Adaptive spatiotemporal SVD clutter filtering for ultrafast Doppler imaging using similarity of spatial singular vectors. IEEE Trans. Med. Imaging 37, 1574–1586 (2018).
Christensen-Jeffries, K., Browning, R. J., Tang, M.-X., Dunsby, C. & Eckersley, R. J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 34, 433–440 (2015).
Demeulenaere, O. et al. In vivo whole brain microvascular imaging in mice using transcranial 3D ultrasound localization microscopy. eBioMedicine 79, 103995 (2022).
Zhou, Q. et al. Cortex-wide transcranial localization microscopy with fluorescently labeled red blood cells. Nat. Commun. 15, 3526 (2024).
Zhou, Q. et al. Depth-resolved localization microangiography in the NIR-II window. Adv. Sci. 10, 2204782 (2023).
Zhou, Q. et al. Three-dimensional wide-field fluorescence microscopy for transcranial mapping of cortical microcirculation. Nat. Commun. 13, 7969 (2022).
Nozdriukhin, D. et al. Rapid volumetric optoacoustic tracking of individual microparticles in vivo enabled by a NIR-absorbing gold–carbon shell. ACS Appl. Mater. Interfaces 13, 48423 (2021).
Nozdriukhin, D. et al. Nanoporous submicron gold particles enable nanoparticle-based localization optoacoustic tomography (nanoLOT). Small 20, e2404904 (2024).
Ji, N. Adaptive optical fluorescence microscopy. Nat. Methods 14, 374–380 (2017).
Kwon, Y. et al. Computational conjugate adaptive optics microscopy for longitudinal through-skull imaging of cortical myelin. Nat. Commun. 14, 105 (2023).
Flax, S. W. & O’Donnell, M. Phase-aberration correction using signals from point reflectors and diffuse scatterers: basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 35, 758–767 (1988).
Ng, G. C., Worrell, S. S., Freiburger, P. D. & Trahey, G. E. A comparative evaluation of several algorithms for phase aberration correction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 41, 631–643 (1994).
Mallart, R. & Fink, M. Adaptive focusing in scattering media through sound‐speed inhomogeneities: the van Cittert Zernike approach and focusing criterion. J. Acoust. Soc. Am. 96, 3721–3732 (1994).
Nock, L., Trahey, G. E. & Smith, S. W. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J. Acoust. Soc. Am. 85, 1819–1833 (1989).
Bendjador, H., Deffieux, T. & Tanter, M. The SVD Beamformer: physical principles and application to ultrafast adaptive ultrasound. IEEE Trans. Med. Imaging 39, 3100–3112 (2020).
Lambert, W., Cobus, L. A., Couade, M., Fink, M. & Aubry, A. Reflection matrix approach for quantitative imaging of scattering media. Phys. Rev. X 10, 021048 (2020).
Bureau, F. et al. Three-dimensional ultrasound matrix imaging. Nat. Commun. 14, 6793 (2023).
Osmanski, B.-F., Montaldo, G., Tanter, M. & Fink, M. Aberration correction by time reversal of moving speckle noise. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 59, 1575–1583 (2012).
Ivancevich, N. M. et al. Real-time 3-D contrast-enhanced transcranial ultrasound and aberration correction. Ultrasound Med. Biol. 34, 1387–1395 (2008).
Lindsey, B. D., Nicoletto, H. A., Bennett, E. R., Laskowitz, D. T. & Smith, S. W. 3-D transcranial ultrasound imaging with bilateral phase aberration correction of multiple isoplanatic patches: a pilot human study with microbubble contrast enhancement. Ultrasound Med. Biol. 40, 90–101 (2014).
Robin, J. et al. In vivo adaptive focusing for clinical contrast-enhanced transcranial ultrasound imaging in human. Phys. Med. Biol. 68, 025019 (2023).
Liu, D.-L. & Waag, R. C. A comparison of ultrasonic wavefront distortion and compensation in one-dimensional and two-dimensional apertures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 42, 726–733 (1995).
Holbek, S. et al. Common carotid artery flow measured by 3-D ultrasonic vector flow imaging and validated with magnetic resonance imaging. Ultrasound Med. Biol. 43, 2213–2220 (2017).
Govinahallisathyanarayana, S., Ning, B., Cao, R., Hu, S. & Hossack, J. A. Dictionary learning-based reverberation removal enables depth-resolved photoacoustic microscopy of cortical microvasculature in the mouse brain. Sci. Rep. 8, 985 (2018).
Huang, C. et al. Aberration correction for transcranial photoacoustic tomography of primates employing adjunct image data. J. Biomed. Opt. 17, 066016 (2012).
Treeby, B. E. & Cox, B. T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 15, 021314 (2010).
Bancel, T. et al. Comparison between ray-tracing and full-wave simulation for transcranial ultrasound focusing on a clinical system using the transfer matrix formalism. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 68, 2554–2565 (2021).
Mitsuhashi, K. et al. A forward-adjoint operator pair based on the elastic wave equation for use in transcranial photoacoustic computed tomography. SIAM J. Imaging Sci. 10, 2022–2048 (2017).
Na, S. et al. Transcranial photoacoustic computed tomography based on a layered back-projection method. Photoacoustics 20, 100213 (2020).
Poudel, J. & Anastasio, M. A. Joint reconstruction of initial pressure distribution and spatial distribution of acoustic properties of elastic media with application to transcranial photoacoustic tomography. Inverse Probl. 36, 124007 (2020).
Robertson, J., Martin, E., Cox, B. & Treeby, B. E. Sensitivity of simulated transcranial ultrasound fields to acoustic medium property maps. Phys. Med. Biol. 62, 2559–2580 (2017).
Di Ianni, T. & Airan, R. D. Deep-fUS: a deep learning platform for functional ultrasound imaging of the brain using sparse data. IEEE Trans. Med. Imaging 41, 1813–1825 (2022).
Stanziola, A., Arridge, S. R., Cox, B. T. & Treeby, B. E. A Helmholtz equation solver using unsupervised learning: application to transcranial ultrasound. J. Comput. Phys. 441, 110430 (2021).
Bergel, A. et al. Adaptive modulation of brain hemodynamics across stereotyped running episodes. Nat. Commun. 11, 6193 (2020).
Sans-Dublanc, A. et al. Optogenetic fUSI for brain-wide mapping of neural activity mediating collicular-dependent behaviors. Neuron 109, 1888–1905.e10 (2021).
Gao, Y., Xu, W., Chen, Y., Xie, W. & Cheng, Q. Deep learning-based photoacoustic imaging of vascular network through thick porous media. IEEE Trans. Med. Imaging 41, 2191–2204 (2022).
Estrada, H. et al. Intravital optoacoustic and ultrasound bio-microscopy reveal radiation-inhibited skull angiogenesis. Bone 133, 115251 (2020).
Olefir, I., Merčep, E., Burton, N. C., Ovsepian, S. V. & Ntziachristos, V. Hybrid multispectral optoacoustic and ultrasound tomography for morphological and physiological brain imaging. J. Biomed. Opt. 21, 086005 (2016).
Mercep, E., Ben, X. L. D. & Razansky, D. Combined pulse-echo ultrasound and multispectral optoacoustic tomography with a multi-segment detector array. IEEE Trans. Med. Imaging 36, 2129–2137 (2017).
Tang, Y. et al. Non-invasive deep-brain imaging with 3D integrated photoacoustic tomography and ultrasound localization microscopy (3D-PAULM). IEEE Trans. Med. Imaging 44, 994–1004 (2025).
Meng, Y., Hynynen, K. & Lipsman, N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat. Rev. Neurol. 17, 7–22 (2021).
Schwartz, M. L. et al. Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures. J. Neurosurg. 130, 758–762 (2018).
Landa, F. J. O., Penacoba, S. R., de Espinosa, F. M., Razansky, D. & Deán-Ben, X. L. Four-dimensional optoacoustic monitoring of tissue heating with medium intensity focused ultrasound. Ultrasonics 94, 117–123 (2019).
Arvanitis, C. D. & McDannold, N. Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies. Med. Phys. 40, 112901 (2013).
Chen, K.-T. et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 7, eabd0772 (2021).
Kim, E., Anguluan, E. & Kim, J. G. Monitoring cerebral hemodynamic change during transcranial ultrasound stimulation using optical intrinsic signal imaging. Sci. Rep. 7, 13148 (2017).
Lake, E. M. R. et al. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat. Methods 17, 1262–1271 (2020).
Robin, J. et al. Hemodynamic response to sensory stimulation in mice: comparison between functional ultrasound and optoacoustic imaging. NeuroImage 237, 118111 (2021).
Chen, Z. Simultaneous functional magnetic resonance and optoacoustic imaging of brain-wide sensory responses in mice. Adv. Sci. 10, 2205191 (2023).
Chen, Z. Multi-modal noninvasive functional neurophotonic imaging of murine brain-wide sensory stimulation. Adv. Sci. 9, 2105588 (2022).
Chen, Z. et al. Multimodal optoacoustic imaging: methods and contrast materials. Chem. Soc. Rev. 53, 6068–6099 (2024).
Gezginer, I. et al. Concurrent optoacoustic tomography and magnetic resonance imaging of resting-state functional connectivity in the mouse brain. Nat. Commun. 15, 10791 (2024).
Deffieux, T., Demené, C. & Tanter, M. Functional ultrasound imaging: a new imaging modality for neuroscience. Neuroscience 474, 110–121 (2021).
Hingot, V. et al. Early ultrafast ultrasound imaging of cerebral perfusion correlates with ischemic stroke outcomes and responses to treatment in mice. Theranostics 10, 7480–7491 (2020).
Chavignon, A., Hingot, V., Orset, C., Vivien, D. & Couture, O. 3D transcranial ultrasound localization microscopy for discrimination between ischemic and hemorrhagic stroke in early phase. Sci. Rep. 12, 14607 (2022).
Lowerison, M. R. et al. Aging-related cerebral microvascular changes visualized using ultrasound localization microscopy in the living mouse. Sci. Rep. 12, 619 (2022).
Yu, J., Dong, H., Ta, D., Xie, R. & Xu, K. Super-resolution ultrasound microvascular angiography for spinal cord penumbra imaging. Ultrasound Med. Biol. 49, 2140–2151 (2023).
Wang, Y. et al. Longitudinal awake imaging of deep mouse brain microvasculature with super-resolution ultrasound localization microscopy. eLife 13, RP95168 (2024).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Kim, J. et al. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci. Rep. 6, 35137 (2016).
Shen, C., Xu, J., Fang, N. X. & Jing, Y. Anisotropic complementary acoustic metamaterial for canceling out aberrating layers. Phys. Rev. X 4, 41033 (2014).
Wang, J., Allein, F., Boechler, N., Friend, J. & Vazquez-Mena, O. Design and fabrication of negative-refractive-index metamaterial unit cells for near-megahertz enhanced acoustic transmission in biomedical ultrasound applications. Phys. Rev. Appl. 15, 24025 (2021).
Jiménez-Gambin, S., Jiménez, N., Benlloch, J. M. & Camarena, F. Holograms to focus arbitrary ultrasonic fields through the skull. Phys. Rev. Appl. 12, 14016 (2019).
Maimbourg, G., Houdouin, A., Deffieux, T., Tanter, M. & Aubry, J.-F. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys. Med. Biol. 63, 25026 (2018).
Subochev, P., Orlova, A., Shirmanova, M., Postnikova, A. & Turchin, I. Simultaneous photoacoustic and optically mediated ultrasound microscopy: an in vivo study. Biomed. Opt. Express 6, 631–638 (2015).
Woitzik, J., Peña-Tapia, P. G., Schneider, U. C., Vajkoczy, P. & Thomé, C. Cortical perfusion measurement by indocyanine-green videoangiography in patients undergoing hemicraniectomy for malignant stroke. Stroke 37, 1549–1551 (2006).
Stoffels, I. et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 7, 317ra199 (2015).
Guasch, L., Calderón Agudo, O., Tang, M.-X., Nachev, P. & Warner, M. Full-waveform inversion imaging of the human brain. npj Digit. Med. 3, 28 (2020).
Taskin, U. et al. Ultrasound imaging of the brain using full-waveform inversion. In Proc. 2020 IEEE International Ultrasonics Symposium 1–4 (IEEE, 2020).
Renaudin, N. et al. Functional ultrasound localization microscopy reveals brain-wide neurovascular activity on a microscopic scale. Nat. Methods 19, 1004–1012 (2022).
Borden, M. A., Dayton, P. A., Slagle, C. & Walmer, R. W. in Molecular Imaging 2nd edn (eds Ross, B. D. & Gambhir, S. S.) 639–653 (Academic Press, 2021).
Acknowledgements
We acknowledge support from the Swiss National Science Foundation (grant 310030_192757), Innosuisse—the Swiss Innovation Agency (grant 51767.1 IP-LS), the Personalized Health and Related Technologies grant of the ETH Domain (PHRT-582) and the US National Institutes of Health (grant RF1-NS126102).
Author information
Authors and Affiliations
Contributions
H.E. and D.R. initiated and coordinated the project. D.R. and M.T. conceptualized the manuscript. H.E. designed the figures. All authors performed the literature research, wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Pengfei Song and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Estrada, H., Deffieux, T., Robin, J. et al. Imaging the brain by traversing the skull with light and sound. Nat. Biomed. Eng 9, 1574–1590 (2025). https://doi.org/10.1038/s41551-025-01433-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01433-5
This article is cited by
-
De-aberration for noninvasive transcranial photoacoustic computed tomography through an adult human skull
Communications Physics (2026)