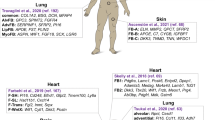
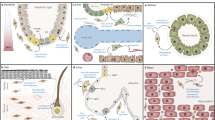
Abstract
Fibroblasts, once perceived as a uniform cell type, are now recognized as a mosaic of distinct populations with specialized roles in tissue homeostasis and pathology. Here we provide a global overview of the expanding compendium of fibroblast cell types and states, their diverse lineage origins and multifaceted functions across various human organs. By integrating insights from developmental biology, lineage tracing and single-cell technologies, we highlight the complex nature of fibroblasts. We delve into their origination from embryonic mesenchyme and tissue-resident populations, elucidating lineage-specific behaviours in response to physiological cues. Furthermore, we highlight the pivotal role of fibroblasts in orchestrating tissue repair, connective tissue remodelling and immune modulation across diverse pathologies. This knowledge is essential to develop novel fibroblast-targeted therapies to restore steady-state fibroblast function and advance regenerative medicine strategies across multiple diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Buechler, M. B. et al. Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579 (2021).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Philippeos, C. et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 138, 811–825 (2018).
LeBleu, V. S. & Neilson, E. G. Origin and functional heterogeneity of fibroblasts. FASEB J. 34, 3519–3536 (2020).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Soundararajan, M. & Kannan, S. Fibroblasts and mesenchymal stem cells: two sides of the same coin? J. Cell. Physiol. 233, 9099–9109 (2018).
Almeida de, D. C. et al. Epigenetic classification of human mesenchymal stromal cells. Stem Cell Rep. 6, 168–175 (2016).
Roson-Burgo, B., Sanchez-Guijo, F., Del Cañizo, C. & De Las Rivas, J. Insights into the human mesenchymal stromal/stem cell identity through integrative transcriptomic profiling. BMC Genom. 17, 944 (2016).
Kumar, A. et al. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 19, 1902–1916 (2017).
Ospelt, C. et al. Expression, regulation, and signaling of the pattern-recognition receptor nucleotide-binding oligomerization domain 2 in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 60, 355–363 (2009).
Nestle, F. O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Miller, L. S. & Cho, J. S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11, 505–518 (2011).
Hinz, B. & Lagares, D. Myofibroblasts (Springer, 2021).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Rivera-Gonzalez, G. C. et al. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. Cell Stem Cell 19, 738–751 (2016).
Maity, P. et al. Persistent JunB activation in fibroblasts disrupts stem cell niche interactions enforcing skin aging. Cell Rep. 36, 109634 (2021).
Kim, B.-C. et al. Fibroblasts from chronic wounds show altered TGF-β-signaling and decreased TGF-β type II receptor expression. J. Cell. Physiol. 195, 331–336 (2003).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Lujano Olazaba, O., Farrow, J. & Monkkonen, T. Fibroblast heterogeneity and functions: insights from single-cell sequencing in wound healing, breast cancer, ovarian cancer and melanoma. Front. Genet. 15, 1304853 (2024).
Kiecker, C., Bates, T. & Bell, E. Molecular specification of germ layers in vertebrate embryos. Cell. Mol. Life Sci. 73, 923–947 (2016).
Usansky, I. et al. A developmental basis for the anatomical diversity of dermis in homeostasis and wound repair. J. Pathol. 253, 315–325 (2021).
Rinkevich, Y. et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151 (2015).
Thulabandu, V., Chen, D. & Atit, R. P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 7, e307 (2018).
Jinno, H. et al. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells 28, 2027–2040 (2010).
Buckingham, M., Meilhac, S. & Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6, 826–835 (2005).
Pærregaard, S. I. et al. The small and large intestine contain related mesenchymal subsets that derive from embryonic Gli1+ precursors. Nat. Commun. 14, 2307 (2023).
Chang, H. Y. et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882 (2002).
Rinn, J. L., Bondre, C., Gladstone, H. B., Brown, P. O. & Chang, H. Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, e119 (2006).
Ganier, C. et al. Multiscale spatial mapping of cell populations across anatomical sites in healthy human skin and basal cell carcinoma. Proc. Natl Acad. Sci. USA 121, e2313326120 (2024).
Koch, C. M. et al. Specific age-associated DNA methylation changes in human dermal fibroblasts. PLoS ONE 6, e16679 (2011).
Rinn, J. L. et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 22, 303–307 (2008).
Thompson, S. M., Phan, Q. M., Winuthayanon, S., Driskell, I. M. & Driskell, R. R. Parallel single-cell multiomics analysis of neonatal skin reveals the transitional fibroblast states that restrict differentiation into distinct fates. J. Invest. Dermatol. 142, 1812–1823.e3 (2022).
Phan, Q. M. et al. Lineage commitment of dermal fibroblast progenitors is controlled by Kdm6b‐mediated chromatin demethylation. EMBO J. 42, e113880 (2023).
Van Camp, J. K., Beckers, S., Zegers, D. & van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. Rep. 10, 207–229 (2014).
Sakaki-Yumoto, M., Katsuno, Y. & Derynck, R. TGF-β family signaling in stem cells. Biochim. Biophys. Acta 1830, 2280–2296 (2013).
Gao, Y. et al. Cross-tissue human fibroblast atlas reveals myofibroblast subtypes with distinct roles in immune modulation. Cancer Cell 42, 1764–1783.e10 (2024).
Driskell, R. R., Jahoda, C. A. B., Chuong, C.-M., Watt, F. M. & Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 23, 629–631 (2014).
Correa-Gallegos, D. et al. CD201+ fascia progenitors choreograph injury repair. Nature 623, 792–802 (2023).
Leavitt, T. et al. Prrx1 fibroblasts represent a pro-fibrotic lineage in the mouse ventral dermis. Cell Rep. 33, 108356 (2020).
Wiedemann, J. et al. Differential cell composition and split epidermal differentiation in human palm, sole, and hip skin. Cell Rep. 42, 111994 (2023).
Marangoni, R. G. et al. Thy-1 plays a pathogenic role and is a potential biomarker for skin fibrosis in scleroderma. JCI Insight 7, e149426 (2022).
Williams, D. W. et al. Human oral mucosa cell atlas reveals a stromal–neutrophil axis regulating tissue immunity. Cell 184, 4090–4104.e15 (2021).
Vorstandlechner, V. et al. The serine proteases dipeptidyl-peptidase 4 and urokinase are key molecules in human and mouse scar formation. Nat. Commun. 12, 6242 (2021).
Korosec, A. et al. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Invest. Dermatol. 139, 342–351 (2019).
Tabib, T., Morse, C., Wang, T., Chen, W. & Lafyatis, R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J. Invest. Dermatol. 138, 802–810 (2018).
Haydont, V. et al. Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling. Cells 9, 368 (2020).
Solé-Boldo, L. et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun. Biol. 3, 188 (2020).
Takaya, K., Asou, T. & Kishi, K. Identification of apolipoprotein D as a dermal fibroblast marker of human aging for development of skin rejuvenation therapy. Rejuvenation Res. 26, 42–50 (2023).
Hsia, C. C. W., Hyde, D. M. & Weibel, E. R. Lung structure and the intrinsic challenges of gas exchange. Compr. Physiol. 6, 827–895 (2016).
Tsukui, T. et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 11, 1920 (2020).
Madissoon, E. et al. A spatially resolved atlas of the human lung characterizes a gland-associated immune niche. Nat. Genet. 55, 66–77 (2023).
Travaglini, K. J. et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587, 619–625 (2020).
Valenzi, E. et al. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann. Rheum. Dis. 78, 1379–1387 (2019).
Pereira, M. S. et al. Loss of SPINT2 expression frequently occurs in glioma, leading to increased growth and invasion via MMP2. Cell. Oncol. 43, 107–121 (2020).
Straus, M. R., Kinder, J. T., Segall, M., Dutch, R. E. & Whittaker, G. R. SPINT2 inhibits proteases involved in activation of both influenza viruses and metapneumoviruses. Virology 543, 43–53 (2020).
Said, S. I., Dey, R. D. & Dickman, K. Glutamate signalling in the lung. Trends Pharmacol. Sci. 22, 344–345 (2001).
Murthy, P. K. L. et al. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature 604, 111–119 (2022).
Xie, T. et al. Abnormal respiratory progenitors in fibrotic lung injury. Stem Cell Res. Ther. 13, 64 (2022).
Habermann, A. C. et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 6, eaba1972 (2020).
El Agha, E. et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20, 261–273.e3 (2017).
Park, J. et al. The Tcf21 lineage constitutes the lung lipofibroblast population. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L872–L885 (2019).
Bowers, S. L. K., Meng, Q. & Molkentin, J. D. Fibroblasts orchestrate cellular crosstalk in the heart through the ECM. Nat. Cardiovasc. Res. 1, 312–321 (2022).
Doll, S. et al. Region and cell-type resolved quantitative proteomic map of the human heart. Nat. Commun. 8, 1469 (2017).
Tallquist, M. D. Cardiac fibroblast diversity. Annu. Rev. Physiol. 82, 63–78 (2020).
Moore-Morris, T., Cattaneo, P., Puceat, M. & Evans, S. M. Origins of cardiac fibroblasts. J. Mol. Cell. Cardiol. 91, 1–5 (2016).
Cui, Y. et al. Single-cell transcriptome analysis maps the developmental track of the human heart. Cell Rep. 26, 1934–1950.e5 (2019).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Passman, J. N. et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc. Natl Acad. Sci. USA 105, 9349–9354 (2008).
Wang, X. et al. Comparative analysis of cell lineage differentiation during hepatogenesis in humans and mice at the single-cell transcriptome level. Cell Res. 30, 1109–1126 (2020).
Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 21, 311–335 (2001).
Andrews, T. S. et al. Single-cell, single-nucleus, and spatial RNA sequencing of the human liver identifies cholangiocyte and mesenchymal heterogeneity. Hepatol. Commun. 6, 821–840 (2022).
Park, H.-J. et al. Cellular heterogeneity and plasticity during NAFLD progression. Front. Mol. Biosci. 10, 1221669 (2023).
Wells, R. G. The portal fibroblast: not just a poor man’s stellate cell. Gastroenterology 147, 41–47 (2014).
Perepelyuk, M. et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G605-14 (2013).
Lua, I. et al. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J. Hepatol. 64, 1137–1146 (2016).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA 109, 9448–9453 (2012).
Elmentaite, R. et al. Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn’s disease. Dev. Cell 55, 771–783.e5 (2020).
Brügger, M. D. & Basler, K. The diverse nature of intestinal fibroblasts in development, homeostasis, and disease. Trends Cell Biol. 33, 834–849 (2023).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178, 714–730.e22 (2019).
Kinchen, J. et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372–386.e17 (2018).
Huang, B. et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell 179, 1160–1176.e24 (2019).
Li, S. et al. An integrated map of fibroblastic populations in human colon mucosa and cancer tissues. Commun. Biol. 5, 1326 (2022).
Knoop, K. A. et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J. Immunol. 183, 5738–5747 (2009).
Nagashima, K. et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 18, 675–682 (2017).
Willemsen, L. E. M., Koetsier, M. A., van Deventer, S. J. H. & van Tol, E. A. F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52, 1442–1447 (2003).
Dorofeyev, A. E., Vasilenko, I. V., Rassokhina, O. A. & Kondratiuk, R. B. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol. Res. Pract. 2013, 431231 (2013).
Larsson, J. M. H. et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 17, 2299–2307 (2011).
Korsunsky, I. et al. Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases. Med. 3, 481–518.e14 (2022).
He, H. et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J. Allergy Clin. Immunol. 145, 1615–1628 (2020).
Huang, X. et al. CD39+ fibroblasts enhance myofibroblast activation by promoting IL-11 secretion in hypertrophic scars. J. Invest. Dermatol. 142, 1065–1076.e19 (2022).
Friedrich, M. et al. IL-1-driven stromal–neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
Yang, D., Liu, J., Qian, H. & Zhuang, Q. Cancer-associated fibroblasts: from basic science to anticancer therapy. Exp. Mol. Med. 55, 1322–1332 (2023).
Gong, Z. et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 55, 1483–1500.e9 (2022).
Younesi, F. S., Miller, A. E., Barker, T. H., Rossi, F. M. V. & Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 25, 617–638 (2024).
Plikus, M. V. et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752 (2017).
Xie, T. et al. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J. Clin. Invest. 126, 3063–3079 (2016).
Talbott, H. E., Mascharak, S., Griffin, M., Wan, D. C. & Longaker, M. T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 29, 1161–1180 (2022).
Correa-Gallegos, D. et al. Patch repair of deep wounds by mobilized fascia. Nature 576, 287–292 (2019).
Dulauroy, S., Di Carlo, S. E., Langa, F., Eberl, G. & Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 (2012).
Kalgudde Gopal, S. et al. Wound infiltrating adipocytes are not myofibroblasts. Nat. Commun. 14, 3020 (2023).
Shook, B. A. et al. Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell 26, 880–895.e6 (2020).
Melms, J. C. et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 595, 114–119 (2021).
Derynck, R. & Budi, E. H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 12, eaav5183 (2019).
Massagué, J. & Sheppard, D. TGF-β signaling in health and disease. Cell 186, 4007–4037 (2023).
Tabib, T. et al. Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat. Commun. 12, 4384 (2021).
Mascharak, S. et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 29, 315–327.e6 (2022).
Currie, J. D. et al. The Prrx1 limb enhancer marks an adult subpopulation of injury-responsive dermal fibroblasts. Biol. Open 8, bio043711 (2019).
Wan, L. et al. Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 97, 58–71 (2021).
Jiang, D. et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 11, 5653 (2020).
Tarzemany, R., Jiang, G., Larjava, H. & Häkkinen, L. Expression and function of connexin 43 in human gingival wound healing and fibroblasts. PLoS ONE 10, e0115524 (2015).
Ezzo, M. et al. Acute contact with profibrotic macrophages mechanically activates fibroblasts via αvβ3 integrin-mediated engagement of Piezo1. Sci. Adv. 10, eadp4726 (2024).
Lodyga, M. et al. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci. Signal. 12, eaao3469 (2019).
Moss, B. J., Ryter, S. W. & Rosas, I. O. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 17, 515–546 (2022).
Koenig, A. L. et al. Single-cell transcriptomics reveals cell-type-specific diversification in human heart failure. Nat. Cardiovasc. Res. 1, 263–280 (2022).
Peisker, F. et al. Mapping the cardiac vascular niche in heart failure. Nat. Commun. 13, 3027 (2022).
Kuppe, C. et al. Spatial multi-omic map of human myocardial infarction. Nature 608, 766–777 (2022).
Rao, M. et al. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res. Cardiol. 116, 55 (2021).
Dobie, R. et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep. 29, 1832–1847.e8 (2019).
Brügger, M. D., Valenta, T., Fazilaty, H., Hausmann, G. & Basler, K. Distinct populations of crypt-associated fibroblasts act as signaling hubs to control colon homeostasis. PLoS Biol. 18, e3001032 (2020).
Correa-Gallegos, D., Jiang, D. & Rinkevich, Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 302, 147–162 (2021).
Deng, C.-C. et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat. Commun. 12, 3709 (2021).
Gur, C. et al. LGR5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell 185, 1373–1388.e20 (2022).
Zhu, H. et al. Fibroblast subpopulations in systemic sclerosis: functional implications of individual subpopulations and correlations with clinical features. J. Invest. Dermatol. 144, 1251–1261.e13 (2024).
Ma, F. et al. Systems-based identification of the Hippo pathway for promoting fibrotic mesenchymal differentiation in systemic sclerosis. Nat. Commun. 15, 210 (2024).
Di Sabatino, A. et al. Transforming growth factor β signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 58, 777–789 (2009).
Ou, W. et al. Increased expression of yes-associated protein/YAP and transcriptional coactivator with PDZ-binding motif/TAZ activates intestinal fibroblasts to promote intestinal obstruction in Crohn’s disease. EBioMedicine 69, 103452 (2021).
Crespi, M., Dulbecco, P., Ceglie de, A. & Conio, M. Strictures in Crohn’s disease: from pathophysiology to treatment. Dig. Dis. Sci. 65, 1904–1916 (2020).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Friščić, J. et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54, 1002–1021.e10 (2021).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791 (2018).
Shang, L., Hosseini, M., Liu, X., Kisseleva, T. & Brenner, D. A. Human hepatic stellate cell isolation and characterization. J. Gastroenterol. 53, 6–17 (2018).
Jiang, D. & Rinkevich, Y. Scars or regeneration? Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 21, 617 (2020).
Gomes, R. N., Manuel, F. & Nascimento, D. S. The bright side of fibroblasts: molecular signature and regenerative cues in major organs. NPJ Regen. Med. 6, 43 (2021).
Brewer, C. M. et al. Adaptations in Hippo–Yap signaling and myofibroblast fate underlie scar-free ear appendage wound healing in spiny mice. Dev. Cell 56, 2722–2740.e6 (2021).
Van Beijnum, H. et al. Spatial transcriptomics reveals asymmetric cellular responses to injury in the regenerating spiny mouse (Acomys) ear. Genome Res. 33, 1424–1437 (2023).
Phan, Q. M., Sinha, S., Biernaskie, J. & Driskell, R. R. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp. Dermatol. 30, 92–101 (2021).
Sinha, S. et al. Fibroblast inflammatory priming determines regenerative versus fibrotic skin repair in reindeer. Cell 185, 4717–4736.e25 (2022).
Foote, A. G., Wang, Z., Kendziorski, C. & Thibeault, S. L. Tissue specific human fibroblast differential expression based on RNAsequencing analysis. BMC Genom. 20, 308 (2019).
Gauthier, V. et al. Fibroblast heterogeneity: keystone of tissue homeostasis and pathology in inflammation and ageing. Front. Immunol. 14, 1137659 (2023).
Klaas, M. et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 6, 27398 (2016).
Eming, S. A., Martin, P. & Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr6 (2014).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001 (2018).
Acknowledgements
This project was funded by the German Federal Ministry of Education and Research under code 03ZU1111GA as part of the Clusters4Future cluster SaxoCell. Y.R. is supported by a European Research Council Consolidator Grant (ERC-CoG 819933), the LEO Foundation (LF-OC-21-000835) and the EFSD Anniversary Fund Programme (CRC/TRR 359; Perinatal Development of Immune Cell Topology (PILOT)). J.C.S. and S.F. are supported by the 3D4D2 project carried out under the M-ERA.NET 2 scheme (the European Union’s Horizon 2020 research and innovation programme; grant number 685451) and co-funded by the Saxon State Ministry for Science, Culture and Tourism (grant number 100579959), as well as tax funds from the Saxon State Parliament. S.F. is supported by the German Research Foundation (FR2671/5-1). Some of the ideas presented in this Review were initiated at a fibroblast symposium at the University of Toronto. We thank B. Hinz, N. Henderson, G. Gabbiani, C. Philippeos, J. Duffield, R. Schwabe and F. Rossi for critical discussions throughout this meeting, as well as S. Miyara for interesting talks on cardiac fibroblasts.
Author information
Authors and Affiliations
Contributions
M.T. wrote the article with guidance from Y.R. and S.F. All authors discussed the manuscript content. M.T., S.F. and Y.R. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Valerie Horsley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torregrossa, M., Davies, L., Hans-Günther, M. et al. Effects of embryonic origin, tissue cues and pathological signals on fibroblast diversity in humans. Nat Cell Biol 27, 720–735 (2025). https://doi.org/10.1038/s41556-025-01638-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01638-5
This article is cited by
-
May In focus in HCB
Histochemistry and Cell Biology (2025)