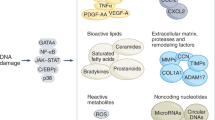
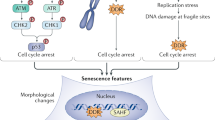
Abstract
Organisms must constantly respond to stress to maintain homeostasis, and the successful implementation of cellular stress responses is directly linked to lifespan regulation. In this Review we examine how three age-associated stressors—loss of proteostasis, oxidative damage and dysregulated nutrient sensing—alter protein synthesis. We describe how these stressors inflict cellular damage via their effects on translation and how translational changes can serve as both sensors and responses to the stressor. Finally, we compare stress-induced translational programmes to protein synthesis alterations that occur with age and discuss whether these changes are adaptive or deleterious to longevity and healthy ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gladyshev, V. N. et al. Molecular damage in aging. Nat. Aging 1, 1096–1106 (2021).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
Morimoto, R. I. Cell-nonautonomous regulation of proteostasis in aging and disease. Cold Spring Harb. Perspect. Biol. 12, a034074 (2020).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Shen, K. et al. Mitochondria as cellular and organismal signaling hubs. Annu. Rev. Cell Dev. Biol. 38, 179–218 (2022).
Wolff, S., Weissman, J. S. & Dillin, A. Differential scales of protein quality control. Cell 157, 52–64 (2014).
Steffen, K. K. & Dillin, A. A ribosomal perspective on proteostasis and aging. Cell Metab. 23, 1004–1012 (2016).
Lane, N. & Martin, W. The energetics of genome complexity. Nature 467, 929–934 (2010).
Shore, D. & Albert, B. Ribosome biogenesis and the cellular energy economy. Curr. Biol. 32, R589–R683 (2022).
Warner, J. R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24, 437–440 (1999).
Storci, G. et al. Ribosomal DNA instability: an evolutionary conserved fuel for inflammaging. Ageing Res. Rev. 58, 101018 (2020).
Lee, J. W. & Ong, E. B. B. Genomic instability and cellular senescence: lessons from the budding yeast. Front. Cell Dev. Biol. 8, 619126 (2021).
Boulon, S., Westman, B. J., Hutten, S., Boisvert, F. M. & Lamond, A. I. The nucleolus under stress. Mol. Cell 40, 216–227 (2010).
Sutandy, F. X. R., Gößner, I., Tascher, G. & Münch, C. A cytosolic surveillance mechanism activates the mitochondrial UPR. Nature 618, 849–854 (2023).
Mühlhofer, M. et al. The heat shock response in yeast maintains protein homeostasis by chaperoning and replenishing proteins. Cell Rep. 29, 4593–4607 (2019).
Shalgi, R. et al. Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 49, 439–452 (2013).
Duncan, R. & Hershey, J. W. B. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J. Biol. Chem. 259, 11882–11889 (1984).
Vries, R. G. J. et al. Heat shock increases the association of binding protein-1 with initiation factor 4E. J. Biol. Chem. 272, 32779–32784 (1997).
Buchan, J. R. & Parker, R. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941 (2009).
Liu, Y., Liang, S. & Tartakoffl, A. M. Heat shock disassembles the nucleolus and inhibits nuclear protein import and poly(A)+ RNA export. EMBO J. 15, 6750–6757 (1996).
Ivanova, E., Berger, A., Scherrer, A., Alkalaeva, E. & Strub, K. Alu RNA regulates the cellular pool of active ribosomes by targeted delivery of SRP9/14 to 40S subunits. Nucleic Acids Res. 43, 2874–2887 (2015).
Berger, A. et al. Direct binding of the Alu binding protein dimer SRP9/14 to 40S ribosomal subunits promotes stress granule formation and is regulated by Alu RNA. Nucleic Acids Res. 42, 11203–11217 (2014).
Bujisic, B. et al. 7SL RNA and signal recognition particle orchestrate a global cellular response to acute thermal stress. Nat. Commun. 16, 1630 (2025).
Liu, B., Han, Y. & Qian, S. B. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol. Cell 49, 453–463 (2013).
Yueh, A. & Schneider, R. J. Translation by ribosome shunting on adenovirus and Hsp70 MRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14, 414–421 (2000).
Hernández, G., Vázquez-Pianzola, P., Sierra, J. M. & Rivera-Pomar, R. Internal ribosome entry site drives cap-independent translation of reaper and heat shock protein 70 mRNAs in Drosophila embryos. RNA 10, 1783–1797 (2004).
Zhou, J. et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015).
Meyer, K. D. et al. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010 (2015).
Pomatto, L. C. D. & Davies, K. J. A. The role of declining adaptive homeostasis in ageing. J. Physiol. 595, 7275–7309 (2017).
Labbadia, J. & Morimoto, R. I. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol. Cell 59, 639–650 (2015).
Meier, S. et al. Pathological tau promotes neuronal damage by impairing ribosomal function and decreasing protein synthesis. J. Neurosci. 36, 957–962 (2016).
Kanekura, K. et al. Poly-dipeptides encoded by the C9ORF72 repeats block global protein translation. Hum. Mol. Genet. 25, 1803–1813 (2016).
Wang, S. & Sun, S. Translation dysregulation in neurodegenerative diseases: a focus on ALS. Mol. Neurodegener. 18, 58 (2023).
Loveland, A. B. et al. Ribosome inhibition by C9ORF72–ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM. Nat. Commun. 13, 2776 (2022).
Moens, T. G. et al. C9orf72 arginine-rich dipeptide proteins interact with ribosomal proteins in vivo to induce a toxic translational arrest that is rescued by eIF1A. Acta Neuropathol. 137, 487–500 (2019).
Aviner, R. et al. Polyglutamine-mediated ribotoxicity disrupts proteostasis and stress responses in Huntington’s disease. Nat. Cell Biol. 26, 892–902 (2024).
Derisbourg, M. J., Hartman, M. D. & Denzel, M. S. Modulating the integrated stress response to slow aging and ameliorate age-related pathology. Nat. Aging 1, 760–768 (2021).
Hussain, S. G. & Ramaiah, K. V. A. Reduced eIF2α phosphorylation and increased proapoptotic proteins in aging. Biochem. Biophys. Res. Commun. 355, 365–370 (2007).
Naidoo, N., Ferber, M., Master, M., Zhu, Y. & Pack, A. I. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J. Neurosci. 28, 6539–6548 (2008).
Ben-Zvi, A., Miller, E. A. & Morimoto, R. I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. 106, 14914–14919 (2009).
Taylor, R. C. & Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447 (2013).
Acosta-Alvear, D., Harnoss, J. M., Walter, P. & Ashkenazi, A. Homeostasis control in health and disease by the unfolded protein response. Nat. Rev. Mol. Cell Biol. 26, 193–212 (2025).
Derisbourg, M. J., Wester, L. E., Baddi, R. & Denzel, M. S. Mutagenesis screen uncovers lifespan extension through integrated stress response inhibition without reduced mRNA translation. Nat. Commun. 12, 1678 (2021).
Oliveira, M. M. et al. Correction of eIF2-dependent defects in brain protein synthesis, synaptic plasticity, and memory in mouse models of Alzheimer’s disease. Sci. Signal. 14, eabc5429 (2021).
Colla, E. et al. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J. Neurosci. 32, 3301–3305 (2012).
Wang, L., Popko, B. & Roos, R. P. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum. Mol. Genet. 23, 2629–2638 (2014).
Saxena, S., Cabuy, E. & Caroni, P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 12, 627–636 (2009).
Krzyzosiak, A. et al. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell 174, 1216–1228 (2018).
Longo, F. et al. Cell-type-specific disruption of PERK–eIF2α signaling in dopaminergic neurons alters motor and cognitive function. Mol. Psychiatry 26, 6427–6450 (2021).
Krukowski, K. et al. Small molecule cognitive enhancer reverses age-related memory decline in mice. eLife 9, e62048 (2020).
Reid, D. W., Chen, Q., Tay, A. S. L., Shenolikar, S. & Nicchitta, C. V. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell 158, 1362–1374 (2014).
Higgins, R. et al. The unfolded protein response triggers site-specific regulatory ubiquitylation of 40S ribosomal proteins. Mol. Cell 59, 35–49 (2015).
Garshott, D. M. et al. iRQC, a surveillance pathway for 40S ribosomal quality control during mRNA translation initiation. Cell Rep. 36, 109642 (2021).
Walther, D. M. et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell 161, 919–932 (2015).
Guan, B. J. et al. A unique ISR program determines cellular responses to chronic stress. Mol. Cell 68, 885–900 (2017).
Lee, A. S. Y., Kranzusch, P. J., Doudna, J. A. & Cate, J. H. D. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 536, 96–99 (2016).
Soto, I. et al. Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes. Genome Biol. 23, 170 (2022).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Wang, X. & Zhang, G. The mitochondrial integrated stress response: a novel approach to anti-aging and pro-longevity. Ageing Res. Rev. 103, 102603 (2025).
Baker, B. M., Nargund, A. M., Sun, T. & Haynes, C. M. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 8, e1002760 (2012).
Anderson, N. S. & Haynes, C. M. Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol. 30, 428–439 (2020).
Fessler, E. et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 579, 433–437 (2020).
Guo, X. et al. Mitochondrial stress is relayed to the cytosol by an OMA1–DELE1–HRI pathway. Nature 579, 427–432 (2020).
Kusuma, F. et al. PKR mediates the mitochondrial unfolded protein response through double-stranded RNA accumulation under mitochondrial stress. Int. J. Mol. Sci. 25, 7738 (2024).
Kim, Y. et al. PKR senses nuclear and mitochondrial signals by interacting with endogenous double-stranded RNAs. Mol. Cell 71, 1051–1063 (2018).
Ladiges, W., Morton, J., Blakely, C. & Gale, M. Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech. Ageing Dev. 114, 123–132 (2000).
Münch, C. & Harper, J. W. Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature 534, 710–713 (2016).
Quirós, P. M. et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 216, 2027–2045 (2017).
Schäfer, J. A., Bozkurt, S., Michaelis, J. B., Klann, K. & Münch, C. Global mitochondrial protein import proteomics reveal distinct regulation by translation and translocation machinery. Mol. Cell 82, 435–446 (2022).
Gehrke, S. et al. PINK1 and parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab. 21, 95–108 (2015).
Wu, Z. et al. Ubiquitination of ABCE1 by NOT4 in response to mitochondrial damage links co-translational quality control to PINK1-directed mitophagy. Cell Metab. 28, 130–144 (2018).
Dai, D. P. et al. Transcriptional mutagenesis mediated by 8-oxoG induces translational errors in mammalian cells. Proc. Natl Acad. Sci. USA 115, 4218–4222 (2018).
Shcherbik, N. & Pestov, D. G. The impact of oxidative stress on ribosomes: from injury to regulation. Cells 8, 1379 (2019).
Ding, Q., Markesbery, W. R., Cecarini, V. & Keller, J. N. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer’s disease. Neurochem. Res. 31, 705–710 (2006).
Willi, J. et al. Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center. Nucleic Acids Res. 46, 1945–1957 (2018).
Shedlovskiy, D., Zinskie, J. A., Gardner, E., Pestov, D. G. & Shcherbik, N. Endonucleolytic cleavage in the expansion segment 7 of 25S rRNA is an early marker of low-level oxidative stress in yeast. J. Biol. Chem. 292, 18469–18485 (2017).
Topf, U. et al. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat. Commun. 9, 324 (2018).
Yang, Y. M. et al. Chaperone-directed ribosome repair after oxidative damage. Mol. Cell 83, 1527–1537 (2023).
Fusco, C. M. et al. Neuronal ribosomes exhibit dynamic and context-dependent exchange of ribosomal proteins. Nat. Commun. 12, 6127 (2021).
Shenton, D. et al. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 281, 29011–29021 (2006).
Gerashchenko, M. V., Lobanov, A. V. & Gladyshev, V. N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc. Natl Acad. Sci. USA 109, 17394–17399 (2012).
Wu, C. C. C., Zinshteyn, B., Wehner, K. A. & Green, R. High-resolution ribosome profiling defines discrete ribosome elongation states and translational regulation during cellular stress. Mol. Cell 73, 959–970 (2019).
Sanchez, M. et al. Cross talk between eIF2α and eEF2 phosphorylation pathways optimizes translational arrest in response to oxidative stress. iScience 20, 466–480 (2019).
Kang, K. R. & Lee, S.-Y. Effect of serum and hydrogen peroxide on the Ca2+/calmodulin-dependent phosphorylation of eukaryotic elongation factor 2(eEF-2) in Chinese hamster ovary cells. Exp. Mol. Med. 33, 198–204 (2001).
Simões, V. et al. Redox-sensitive E2 Rad6 controls cellular response to oxidative stress via K63-linked ubiquitination of ribosomes. Cell Rep. 39, 110860 (2022).
Meydan, S. et al. The ubiquitin conjugase Rad6 mediates ribosome pausing during oxidative stress. Cell Rep. 42, 113359 (2023).
Zhou, Y. et al. Structural impact of K63 ubiquitin on yeast translocating ribosomes under oxidative stress. Proc. Natl Acad. Sci. USA 117, 22157–22166 (2020).
Santos, C. M. et al. Redox control of the deubiquitinating enzyme Ubp2 regulates translation during stress. J. Biol. Chem. 300, 107870 (2024).
Kim, Y. S., Kimball, S. R., Piskounova, E., Begley, T. J. & Hempel, N. Stress response regulation of mRNA translation: Implications for antioxidant enzyme expression in cancer. Proc. Natl Acad. Sci. USA 121, e2317846121 (2024).
Torrent, M., Chalancon, G., de Groot, N. S., Wuster, A. & Madan Babu, M. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci. Signal. 11, eaat6409 (2018).
Schwenzer, H. et al. Oxidative stress triggers selective tRNA retrograde transport in human cells during the integrated stress response. Cell Rep. 26, 3416–3428 (2019).
Yamasaki, S., Ivanov, P., Hu, G. F. & Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 185, 35–42 (2009).
Czech, A., Wende, S., Mörl, M., Pan, T. & Ignatova, Z. Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet. 9, e1003767 (2013).
Ivanov, P., Emara, M. M., Villen, J., Gygi, S. P. & Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 43, 613–623 (2011).
Lyons, S. M. et al. eIF4G has intrinsic G-quadruplex binding activity that is required for tiRNA function. Nucleic Acids Res. 48, 6223–6233 (2021).
Chan, C. T. Y. et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 3, 937 (2012).
Fernández-Vázquez, J. et al. Modification of tRNALysUUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet. 9, e1003647 (2013).
Huber, S. M. et al. Arsenite toxicity is regulated by queuine availability and oxidation-induced reprogramming of the human tRNA epitranscriptome. Proc. Natl Acad. Sci. USA 119, e2123529119 (2022).
Endres, L. et al. Alkbh8 regulates selenocysteine-protein expression to protect against reactive oxygen species damage. PLoS ONE 10, e0131335 (2015).
Leonardi, A., Evke, S., Lee, M., Melendez, J. A. & Begley, T. J. Epitranscriptomic systems regulate the translation of reactive oxygen species detoxifying and disease linked selenoproteins. Free Radic. Biol. Med. 143, 573–593 (2019).
Li, W. et al. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 38, 778–788 (2009).
Zhang, J., Dinh, T. N., Kappeler, K., Tsaprailis, G. & Chen, Q. M. La autoantigen mediates oxidant induced de novo Nrf2 protein translation. Mol. Cell. Proteom. 11, M111.015032 (2012).
Lee, S. C. et al. G-quadruplex in the NRF2 mRNA 5′ untranslated region regulates de novo NRF2 protein translation under oxidative stress. Mol. Cell. Biol. 37, e00122-16 (2017).
Jennings, M. D. et al. Interaction of the La-related protein Slf1 with colliding ribosomes maintains translation of oxidative-stress responsive mRNAs. Nucleic Acids Res. 51, 5755–5773 (2023).
Timme-Laragy, A. R., Hahn, M. E., Hansen, J. M., Rastogi, A. & Roy, M. A. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Semin. Cell Dev. Biol. 80, 17–28 (2018).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Oleson, B. J., Bazopoulou, D. & Jakob, U. Shaping longevity early in life: developmental ROS and H3K4me3 set the clock. Cell Cycle 20 2337–2347 (2021).
Bazopoulou, D. et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 576, 301–305 (2019).
Lee, J. Y. et al. Promiscuous methionyl-tRNA synthetase mediates adaptive mistranslation to protect cells against oxidative stress. J. Cell Sci. 127, 4234–4245 (2014).
Luo, S. & Levine, R. L. Methionine in proteins defends against oxidative stress. FASEB J. 23, 464–472 (2009).
Lee, H. & Lee, S. J. V. Recent progress in regulation of aging by insulin/IGF-1 signaling in Caenorhabditis elegans. Mol. Cells 45, 763–770 (2022).
Li, W. J. et al. Insulin signaling regulates longevity through protein phosphorylation in Caenorhabditis elegans. Nat. Commun. 12, 4568 (2021).
Stout, G. J. et al. Insulin/IGF-1-mediated longevity is marked by reduced protein metabolism. Mol. Syst. Biol. 9, 679 (2013).
Papadopoli, D. et al. mTOR as a central regulator of lifespan and aging. F1000Res. 8, 998 (2019).
Wu, C. C. C., Peterson, A., Zinshteyn, B., Regot, S. & Green, R. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182, 404–416 (2020).
Coria, A. R. et al. The integrated stress response regulates 18S nonfunctional rRNA decay in mammals. Mol. Cell 85, 787–801 (2025).
Huang, Z. et al. RIOK3 mediates the degradation of 40S ribosomes. Mol. Cell 85, 802–814 (2025).
Ford, P. W. et al. RNF10 and RIOK3 facilitate 40S ribosomal subunit degradation upon 60S biogenesis disruption or amino acid starvation. Cell Rep. 44, 115371 (2025).
Parkhitko, A. A., Filine, E., Mohr, S. E., Moskalev, A. & Perrimon, N. Targeting metabolic pathways for extension of lifespan and healthspan across multiple species. Ageing Res. Rev. 64, 101188 (2020).
Darnell, A. M., Subramaniam, A. R. & O’Shea, E. K. Translational control through differential ribosome pausing during amino acid limitation in mammalian cells. Mol. Cell 71, 229–243 (2018).
Mazor, K. M. et al. Effects of single amino acid deficiency on mRNA translation are markedly different for methionine versus leucine. Sci. Rep. 8, 8076 (2018).
Stein, K. C., Morales-Polanco, F., van der Lienden, J., Rainbolt, T. K. & Frydman, J. Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis. Nature 601, 637–642 (2022).
Singh, P. et al. Taurine deficiency as a driver of aging. Science 380, eabn9257 (2023).
Suzuki, T., Suzuki, T., Wada, T., Saigo, K. & Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 21, 6581–6589 (2002).
Saini, P., Eyler, D. E., Green, R. & Dever, T. E. Hypusine-containing protein eIF5A promotes translation elongation. Nature 459, 118–121 (2009).
Melnikov, S. et al. Crystal structure of hypusine-containing translation factor eIF5A bound to a rotated eukaryotic ribosome. J. Mol. Biol. 428, 3570–3576 (2016).
Schmidt, C. et al. Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 44, 1944–1951 (2015).
Liang, Y. T. et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 35, 108941 (2021).
Gupta, V. K. et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460 (2013).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Schuller, A. P., Wu, C. C. C., Dever, T. E., Buskirk, A. R. & Green, R. eIF5A functions globally in translation elongation and termination. Mol. Cell 66, 194–205 (2017).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125 (2019).
Lubas, M. et al. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 19, e46072 (2018).
Hofer, S. J. et al. Spermidine is essential for fasting-mediated autophagy and longevity. Nat. Cell Biol. 26, 1571–1584 (2024).
Metur, S. P. et al. Yeast TIA1 coordinates with Npl3 to promote ATG1 translation during starvation. Cell Rep. 44, 115316 (2025).
De, S., Das, S. & Sengupta, S. Involvement of HuR in the serum starvation induced autophagy through regulation of Beclin1 in breast cancer cell-line, MCF-7. Cell Signal. 61, 78–85 (2019).
Ji, E. et al. RNA binding protein HuR promotes autophagosome formation by regulating expression of autophagy-related proteins 5, 12, and 16 in human hepatocellular carcinoma cells. Mol. Cell. Biol. 39, e00508–e00518 (2019).
Kim, C. et al. The RNA-binding protein HuD regulates autophagosome formation in pancreatic β cells by promoting autophagy-related gene 5 expression. J. Biol. Chem. 289, 112–121 (2014).
Liu, X. et al. Dhh1 promotes autophagy-related protein translation during nitrogen starvation. PLoS Biol. 17, e3000219 (2019).
Hu, G. et al. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat. Cell Biol. 17, 930–942 (2015).
Kim, H. S. & Pickering, A. M. Protein translation paradox: implications in translational regulation of aging. Front. Cell Dev. Biol. 11, 1129281 (2023).
Baar, E. L., Carbajal, K. A., Ong, I. M. & Lamming, D. W. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 15, 155–166 (2016).
Blagosklonny, M. V. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 9, 3171–3176 (2010).
Anisimova, A. S. et al. Multifaceted deregulation of gene expression and protein synthesis with age. Proc. Natl Acad. Sci. USA 117, 15581–15590 (2020).
Amirbeigiarab, S. et al. Invariable stoichiometry of ribosomal proteins in mouse brain tissues with aging. Proc. Natl Acad. Sci. USA 116, 22567–22572 (2019).
Gerashchenko, M. V., Peterfi, Z., Yim, S. H. & Gladyshev, V. N. Translation elongation rate varies among organs and decreases with age. Nucleic Acids Res. 49, e9 (2021).
Conn, C. S. & Qian, S.-B. Nutrient signaling in protein homeostasis: an increase in quantity at the expense of quality. Sci. Signal. 6, ra24 (2013).
Buhr, F. et al. Synonymous codons direct cotranslational folding toward different protein conformations. Mol. Cell 61, 341–351 (2016).
Sudmant, P. H., Lee, H., Dominguez, D., Heiman, M. & Burge, C. B. Widespread accumulation of ribosome-associated isolated 3′ UTRs in neuronal cell populations of the aging brain. Cell Rep. 25, 2447–2456 (2018).
Kirstein-Miles, J., Scior, A., Deuerling, E. & Morimoto, R. I. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 32, 1451–1468 (2013).
Lu, J. & Deutsch, C. Electrostatics in the ribosomal tunnel modulate chain elongation rates. J. Mol. Biol. 384, 73–86 (2008).
Rieckher, M., Markaki, M., Princz, A., Schumacher, B. & Tavernarakis, N. Maintenance of proteostasis by P body-mediated regulation of eIF4E availability during aging in Caenorhabditis elegans. Cell Rep. 25, 199–211 (2018).
Ding, Q., Markesbery, W. R., Chen, Q., Li, F. & Keller, J. N. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 25, 9171–9175 (2005).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Sacramento, E. K. et al. Reduced proteasome activity in the aging brain results in ribosome stoichiometry loss and aggregation. Mol. Syst. Biol. 16, e9596 (2020).
VanInsberghe, M., van den Berg, J., Andersson-Rolf, A., Clevers, H. & van Oudenaarden, A. Single-cell Ribo-seq reveals cell cycle-dependent translational pausing. Nature 597, 561–565 (2021).
Ozadam, H. et al. Single-cell quantification of ribosome occupancy in early mouse development. Nature 618, 1057–1064 (2023).
Xiong, Z. et al. Ultrasensitive Ribo-seq reveals translational landscapes during mammalian oocyte-to-embryo transition and pre-implantation development. Nat. Cell Biol. 24, 968–980 (2022).
Gemmer, M. et al. Visualization of translation and protein biogenesis at the ER membrane. Nature 614, 160–167 (2023).
Schaffer, M. et al. A cryo-FIB lift-out technique enables molecular-resolution cryo-ET within native Caenorhabditis elegans tissue. Nat. Methods 16, 757–762 (2019).
Xing, H. et al. Translation dynamics in human cells visualized at high resolution reveal cancer drug action. Science 381, 70–75 (2023).
Zeng, H. et al. Spatially resolved single-cell translatomics at molecular resolution. Science 380, eadd3067 (2023).
Mordret, E. et al. Systematic detection of amino acid substitutions in proteomes reveals mechanistic basis of ribosome errors and selection for translation fidelity. Mol. Cell 75, 427–441 (2019).
Hinnebusch, A. G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 83, 779–812 (2014).
Dever, T. E., Dinman, J. D. & Green, R. Translation elongation and recoding in eukaryotes. Cold Spring Harb. Perspect. Biol. 10, a032649 (2018).
Gutierrez, E. et al. eif5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45 (2013).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Filer, D. et al. RNA polymerase III limits longevity downstream of TORC1. Nature 552, 263–267 (2017).
Roux, P. P. & Topisirovic, I. Signaling pathways involved in the regulation of mRNA translation. Mol. Cell. Biol. 38, e00070-18 (2018).
Zid, B. M. et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139, 149–160 (2009).
Jin, H. et al. TRIBE editing reveals specific mRNA targets of EIF4E–BP in Drosophila and in mammals. Sci. Adv. 6, eabb8771 (2020).
Martinez-Miguel, V. E. et al. Increased fidelity of protein synthesis extends lifespan. Cell Metab. 33, 2288–2300 (2021).
Guan, B.-J. et al. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eif2α. J. Biol. Chem. 289, 12593–12611 (2014).
Park, Y., Reyna-Neyra, A., Philippe, L. & Thoreen, C. C. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep. 19, 1083–1090 (2017).
Torrence, M. E. et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. eLife 10, e63326 (2021).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Ye, J. et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 29, 2331–2336 (2015).
Harris, D. T. & Jan, C. H. CRISPuRe-seq: pooled screening of barcoded ribonucleoprotein reporters reveals regulation of RNA polymerase III transcription by the integrated stress response via mTOR. Nucleic Acids Res. 53, gkaf062 (2025).
Acknowledgements
We thank all members of the Dillin laboratory for their constructive discussion. Figures were created in BioRender. A.D. is supported by the Howard Hughes Medical Institute and N.R.G. is a Howard Hughes Medical Institute Fellow of the Jane Coffin Childs Memorial Fund.
Author information
Authors and Affiliations
Contributions
N.R.G. prepared the manuscript and figures with guidance from A.D. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Genuth, N.R., Dillin, A. Translational regulation in stress biology. Nat Cell Biol (2025). https://doi.org/10.1038/s41556-025-01765-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41556-025-01765-z