Abstract
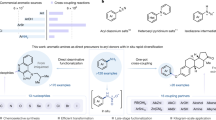
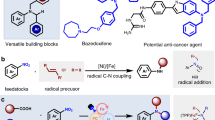
Nitroarenes are readily accessible bulk chemicals and can serve as versatile starting materials for a series of synthetic reactions. However, due to the inertness of the CAr–NO2 bond, the direct denitrative substitution reaction with unactivated nitroarenes remains challenging. Chemists rely on sequential reduction and diazotization followed by the Sandmeyer reaction or the nucleophilic aromatic substitution of activated nitroarenes to realize nitro group transformations. Here we develop a general denitrative chlorination reaction under visible-light irradiation, in which the chlorine radical replaces the nitro moiety through the cleavage of the CAr–NO2 bond. This practical method works with a wide range of unactivated nitro(hetero)arenes and nitroalkenes, is not sensitive to air or moisture and can proceed smoothly on a decagram scale. This transformation differs fundamentally from previous nucleophilic aromatic substitution reactions under thermal conditions in both synthesis and mechanism. Density functional theory calculations reveal the possible pathway for the substitution reaction.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compound 52 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2341039. These data can be obtained free of charge from the CCDC (http://www.ccdc.cam.ac.uk/data_request/cif).
References
Ju, K. S. & Parales, R. E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 74, 250–272 (2010).
Booth, G. Nitro Compounds, Aromatic (Wiley-VCH Verlag, 2000).
Sharma, K. Nitro Compounds: Classification, Preparation, Properties, Reactions, Uses (Wiley, 2023).
Amini, B. & Lowenkron, S. Aniline and its derivatives. Kirk-Othmer Encyclopedia of Chemical Technology Vol. 2, 783−809 (Wiley, 2003).
Olah, G. A., Malhotra, R. & Narang, S. C. Nitration: Methods and Mechanism 975–979 (World Scientific, 2003).
Nykaza, T. V. et al. Intermolecular reductive C–N cross coupling of nitroarenes and boronic acids by PIII/PV=O catalysis. J. Am. Chem. Soc. 140, 15200–15205 (2018).
Nykaza, T. V., Ramirez, A., Harrison, T. S., Luzung, M. R. & Radosevich, A.T. Biphilic organophosphorus-catalyzed intramolecular \({{\mathrm{C}}_{{\mathrm{sp}}^{2}}}{-}{\mathrm{H}}\) amination: evidence for a nitrenoid in catalytic cadogan cyclizations. J. Am.Chem. Soc. 140, 3103–3113 (2018).
Nykaza, T. V., Li, G., Yang, J., Luzung, M. R. & Radosevich, A. T. PIII/PV=O catalyzed cascade synthesis of N-functionalized azaheterocycles. Angew. Chem. Int. Ed. 59, 4505–4510 (2020).
Li, G., Lavagnino, M. N., Ali, S. Z., Hu, S. & Radosevich, A. T. Tandem C/N-difunctionalization of nitroarenes: reductive amination and annulation by a ring expansion/contraction sequence. J. Am. Chem. Soc. 145, 41–46 (2023).
Ryabchuk, P. et al. Cascade synthesis of pyrroles from nitroarenes with benign reductants using a heterogeneous cobalt catalyst. Angew. Chem. Int. Ed. 59, 18679–18685 (2020).
Gui, J. et al. Practical olefin hydroamination with nitroarenes. Science 348, 886–891 (2015).
Schwob, T. & Kempe, R. A reusable Co catalyst for the selective hydrogenation of functionalized nitroarenes and the direct synthesis of imines and benzimidazoles from nitroarenes and aldehydes. Angew. Chem. Int. Ed. 55, 15175–15179 (2016).
Gkizis, P. L., Triandafillidi, I. & Kokotos, C. G. Nitroarenes: the rediscovery of their photochemistry opens new avenues in organic synthesis. Chem 9, 3401–3414 (2023).
Paolillo, J. M., Duke, A. D., Gogarnoiu, E. S., Wise, D. E. & Parasram, M. Anaerobic hydroxylation of C(sp3)–H bonds enabled by the synergistic nature of photoexcited nitroarenes. J. Am. Chem. Soc. 145, 2794–2799 (2023).
Ruffoni, A., Hampton, C., Simonetti, M. & Leonori, D. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022).
Hampton, C., Simonetti, M. & Leonori, D. Olefin dihydroxylation using nitroarenes as photoresponsive oxidants. Angew. Chem. Int. Ed. 62, e202214508 (2023).
Wise, D. E. et al. Photoinduced oxygen transfer using nitroarenes for the anaerobic cleavage of alkenes. J. Am. Chem. Soc. 144, 15437–15442 (2022).
Sanchez-Bento, R., Roure, B., Llaveria, J., Ruffoni, A. & Leonori, D. A strategy for ortho-phenylenediamine synthesis via dearomative-rearomative coupling of nitrobenzenes and amines. Chem 9, 3685–3695 (2023).
Li, B., Ruffoni, A. & Leonori, D. A photochemical strategy for ortho-aminophenol synthesis via dearomative–rearomative coupling between aryl azides and alcohols. Angew. Chem. Int. Ed. 62, e202310540 (2023).
Matador, E. et al. A photochemical strategy for the conversion of nitroarenes into rigidified pyrrolidine analogues. J. Am. Chem. Soc. 145, 27810–27820 (2023).
Mykura, R. et al. Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes. Nat. Chem. 16, 771–779 (2024).
Muto, K., Okita, T. & Yamaguchi, J. Transition-metal-catalyzed denitrative coupling of nitroarenes. ACS Catal. 10, 9856–9871 (2020).
Mo, F., Qiu, D., Zhang, Y. & Wang, J. Renaissance of Sandmeyer-type reactions: conversion of aromatic C–N bonds into C–X bonds (X = B, Sn, P, or CF3). Acc. Chem. Res. 51, 496–506 (2018).
Mateos, J. et al. Nitrate reduction enables safer aryldiazonium chemistry. Science 384, 446–452 (2024).
Bunnett, J. F. & Zahler, R. E. Aromatic nucleophilic substitution reactions. Chem. Rev. 49, 273–412 (1951).
Yadav, M. R. et al. The Suzuki–Miyaura coupling of nitroarenes. J. Am. Chem. Soc. 139, 9423–9426 (2017).
Inoue, F., Kashihara, M., Yadav, M. R. & Nakao, Y. Buchwald–Hartwig amination of nitroarenes. Angew. Chem. Int. Ed. 56, 13307–13309 (2017).
Kashihara, M. & Nakao, Y. Cross-coupling reactions of nitroarenes. Acc. Chem. Res. 54, 2928–2935 (2021).
Fráter, G. & Havinga, E. Photosubstitution reactions of nitronaphtalenes leading to chloronaphtalene. Tetrahedron Lett. 10, 4603–4604 (1969).
Vink, I. A. J., Verheijdt, P. L., Cornelisse, J. & Havinga, E. Photoreactions of aromatic compounds—XXVI: photoinduced reactions of biphenyl and biphenyl derivatives with cyanide ion. Tetrahedron 28, 5081–5087 (1972).
Pistritto, V. A., Liu, S. & Nicewicz, D. A. Mechanistic investigation into amination of unactivated arene via cation radical accelerated nucleophilic aromatic substitution. J. Am. Chem. Soc. 144, 15118–15131 (2022).
Pistritto, V. A., Schutzbach-Horton, M. E. & Nicewicz, D. A. Nucleophilic aromatic substitution of unactivated fluoroarenes enabled by organic photoredox catalysis. J. Am. Chem. Soc. 142, 17187–17194 (2020).
Tay, N. E. S. et al. 19F- and 18F-arene deoxyfluorination via organic photoredox-catalysed polarity-reversed nucleophilic aromatic substitution. Nat. Catal. 3, 734–742 (2020).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Juliá, F. Ligand-to-metal charge transfer (LMCT) photochemistry at 3d-metal complexes: an emerging tool for sustainable organic synthesis. ChemCatChem 14, e202200916 (2022).
Song, S. et al. DMSO-catalysed late-stage chlorination of (hetero)arenes. Nat. Catal. 3, 107–115 (2020).
Camelio, A. M. et al. Computational and experimental studies of phthaloyl peroxide-mediated hydroxylation of arenes yield a more reactive derivative, 4,5-dichlorophthaloyl peroxide. J. Org. Chem. 80, 8084–8095 (2015).
Mo, F. et al. Gold catalyzed halogenation of aromatics by N-halosuccinimides. Angew. Chem. Int. Ed. 49, 2028–2032 (2010).
Fosu, S. C., Hambira, C. M., Chen, A. D., Fuchs, J. R. & Nagib, D. A. Site-selective C–H functionalization of (hetero)arenes via transient, non-symmetric iodanes. Chem 5, 417–428 (2019).
Wang, W. et al. Catalytic electrophilic halogenation of arenes with electron-withdrawing substitutents. J. Am. Chem. Soc. 144, 13415–13425 (2022).
da Petruci, J. F. S., Tütüncü, E., Cardos, A. A. & Mizaikoff, B. Real-time and simultaneous monitoring of NO, NO2, and N2O using substrate–integrated hollow waveguides coupled to a compact fourier transform infrared (FT-IR) spectrometer. Appl. Spectrosc. 73, 98–103 (2019).
Tu, J., Hu, A., Guo, L. & Xia, W. Iron-catalyzed C(sp3)–H borylation, thiolation, and sulfinylation enabled by photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 145, 7600–7611 (2023).
Steube, J. et al. Janus-type emission from a cyclometalated iron(III) complex. Nat. Chem. 15, 468–474 (2023).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem. Int. Ed. 60, 21100–21115 (2021).
Chinchole, A., Henriquez, M. A., Cortes-Arriagada, D., Cabrera, A. R. & Reiser, O. Iron(III)-light-induced homolysis: a dual photocatalytic approach for the hydroacylation of alkenes using acyl radicals via direct HAT from aldehydes. ACS Catal. 12, 13549–13554 (2022).
Acknowledgements
We thank J. Wang (PKU) for the high-resolution MS testing and Z. Luo (Central China Normal University (CCNU)) for the IR testing. We thank X. Meng (CCNU) for X-ray structure refinement. We thank L. Chen (CCNU) for electron paramagnetic resonance measurement. We also thank Y. Xu (Peking University) and G. Wu (Huazhong University of Science and Technology) for helpful discussion about the project. We are grateful to the Knowledge Innovation Program of the Wuhan-Shuguang Project (2023020201020308; F.Y.); the Cultivation Program of Wuhan Institute of Photochemistry and Technology (GHY2023KF003; F.Y.); the National Natural Science Foundation of China (22173077 and 22422110; G.-J.C.) and the Guangdong Basic and Applied Basic Research Foundation (2023B1515020052; G.-J.C.) for financial support; and CCNU for startup funding (F.Y.).
Author information
Authors and Affiliations
Contributions
T.L. developed the reaction methods and investigated the mechanism. T.L., Y.W. and W.Z. explored the substrate scope. Z.L. performed the DFT calculation. R.S, G.-J.C. and F.Y. prepared the paper. F.Y. directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Wei Guan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Tables 1–4, Discussion, experimental data, procedural details, synthesis and characterization data and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 52; CCDC reference number 2341039.
Supplementary Data 2
Calculated coordinates of intermediates and transition states.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, T., Lyu, Z., Wang, Y. et al. Light-promoted aromatic denitrative chlorination. Nat. Chem. 17, 598–605 (2025). https://doi.org/10.1038/s41557-024-01728-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01728-1