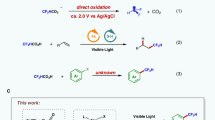
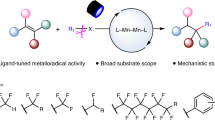
Abstract
Selective activation of specific C–F bonds in polyfluoroarenes represents a challenge in transition metal catalysis. Here we report a photoexcited nickel-catalysed cross-electrophile coupling between polyfluoroarenes and aryl chlorides, achieving highly selective arylation at atypical C–F sites of fluoroarenes, facilitated by a synergistic lithium salt effect. A wide range of structurally diverse fluorine-containing biaryls are obtained in 33−94% yields with satisfying C–F regioselectivity. Notably, the observed regioselectivity is atypical and complements existing methodologies, such as palladium-catalysed and visible-light photoredox-catalysed defluorinative functionalization reactions. Our mechanistic studies and theoretical calculations suggest that the lithium salt could interact with both pentafluorobenzene and the Ni catalyst, effectively lowering the energy barrier and modulating the regioselectivity. The synthetic versatility of our approach is underscored by late-stage synthetic application and sequential functionalization of multiple C–F bonds, which further demonstrates its robust utility in concisely constructing partially fluorinated and biologically interesting compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2231838 (13), 2231820 (14), 2231850 (16), 2231848 (17), 2231841 (20), 2231844 (25) and 2231843 (79). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. All other data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496–3508 (2011).
Li, M., Xue, X. S. & Cheng, J. P. Establishing cation and radical donor ability scales of electrophilic F, CF3, and SCF3 transfer reagents. Acc. Chem. Res. 53, 182–197 (2020).
Xiao, P., Pannecoucke, X., Bouillon, J. P. & Couve-Bonnaire, S. Wonderful fusion of organofluorine chemistry and decarboxylation strategy. Chem. Soc. Rev. 50, 6094–6151 (2021).
He, M., Soulé, J.-F. & Doucet, H. Synthesis of (poly)fluorobiphenyls through metal-catalyzed C−H bond activation/arylation of (poly)fluorobenzene derivatives. ChemCatChem 6, 1824–1859 (2014).
He, C.-Y., Fan, S. & Zhang, X. Pd-catalyzed oxidative cross-coupling of perfluoroarenes with aromatic heterocycles. J. Am. Chem. Soc. 132, 12850–12852 (2010).
Amii, H. & Uneyama, K. C–F bond activation in organic synthesis. Chem. Rev. 109, 2119–2183 (2009).
Eisenstein, O., Milani, J. & Perutz, R. N. Selectivity of C–H activation and competition between C–H and C–F bond activation at fluorocarbons. Chem. Rev. 117, 8710–8753 (2017).
Senaweera, S. M., Singh, A. & Weaver, J. D. Photocatalytic hydrodefluorination: facile access to partially fluorinated aromatics. J. Am. Chem. Soc. 136, 3002–3005 (2014).
Chen, Y. J. et al. Transition-metal-free, site-selective C–F arylation of polyfluoroarenes via electrophotocatalysis. J. Am. Chem. Soc. 144, 17261–17268 (2022).
Arora, A. & Weaver, J. D. Visible light photocatalysis for the generation and use of reactive azolyl and polyfluoroaryl intermediates. Acc. Chem. Res. 49, 2273–2283 (2016).
Qin, J., Zhu, S. Q. & Chu, L. L. Dual photoredox-/palladium-catalyzed cross-electrophile couplings of polyfluoroarenes with aryl halides and triflates. Organometallics 40, 2246–2252 (2021).
Dewanji, A., Bulow, R. F. & Rueping, M. Photoredox/nickel dual-catalyzed reductive cross coupling of aryl halides using an organic reducing agent. Org. Lett. 22, 1611–1617 (2020).
Senaweera, S. & Weaver, J. D. Dual C–F, C–H functionalization via photocatalysis: access to multifluorinated biaryls. J. Am. Chem. Soc. 138, 2520–2523 (2016).
Ahrens, T., Kohlmann, J., Ahrens, M. & Braun, T. Functionalization of fluorinated molecules by transition-metal-mediated C–F bond activation to access fluorinated building blocks. Chem. Rev. 115, 931–972 (2015).
Diccianni, J. B. & Diao, T. N. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 1, 830–844 (2019).
Liu, X. W., Echavarren, J., Zarate, C. & Martin, R. Ni-catalyzed borylation of aryl fluorides via C–F cleavage. J. Am. Chem. Soc. 137, 12470–12473 (2015).
Zhou, J. et al. Preparing (multi)fluoroarenes as building blocks for synthesis: nickel-catalyzed borylation of polyfluoroarenes via C–F bond cleavage. J. Am. Chem. Soc. 138, 5250–5253 (2016).
Schaub, T. et al. C−F activation of fluorinated arenes using NHC-stabilized nickel(0) complexes: selectivity and mechanistic investigations. J. Am. Chem. Soc. 130, 9304–9317 (2008).
Nakamura, Y., Yoshikai, N., Ilies, L. & Nakamura, E. Nickel-catalyzed monosubstitution of polyfluoroarenes with organozinc reagents using alkoxydiphosphine ligand. Org. Lett. 14, 3316–3319 (2012).
Clot, E. et al. C−F and C−H bond activation of fluorobenzenes and fluoropyridines at transition metal centers: how fluorine tips the scales. Acc. Chem. Res. 44, 333–348 (2011).
Schaub, T., Backes, M. & Radius, U. Square-planar (pentafluorophenyl)nickel(II) complexes by derivatization of a C–F activation product. Eur. J. Inorg. Chem. 17, 2680–2690 (2008).
Braun, T. & Perutz, R. N. Routes to fluorinated organic derivatives by nickel mediated C–F activation of heteroaromatics. Chem. Commun. 2749−2757 (2002).
Wang, K. & Kong, W. Synthesis of fluorinated compounds by nickel-catalyzed defluorinative cross-coupling reactions. ACS Catal. 13, 12238–12268 (2023).
Ren, J.-A. et al. Nickel-catalyzed direct cross-coupling of unactivated aryl fluorides with aryl bromides. Org. Lett. 25, 5525–5529 (2023).
Das, A. & Chatani, N. The directing group: a tool for efficient and selective C–F bond activation. ACS Catal. 11, 12915–12930 (2021).
Sun, A. D. & Love, J. A. Nickel-catalyzed selective defluorination to generate partially fluorinated biaryls. Org. Lett. 13, 2750–2753 (2011).
Capdevila, L. et al. Chemodivergent nickel(0)-catalyzed arene C–F activation with alkynes: unprecedented C–F/C–H double insertion. ACS Catal. 9, 11074–11081 (2019).
Kuntze-Fechner, M. W. et al. Coligand role in the NHC nickel catalyzed C–F bond activation: investigations on the insertion of bis(NHC) nickel into the C–F bond of hexafluorobenzene. Chem. Sci. 11, 11009–11023 (2020).
Doster, M. E. & Johnson, S. A. Selective C–F bond activation of tetrafluorobenzenes by nickel(0) with a nitrogen donor analogous to N-heterocyclic carbenes. Angew. Chem. Int. Ed. 48, 2185–2187 (2009).
Doster, M. E., Hatnean, J. A., Jeftic, T., Modi, S. & Johnson, S. A. Catalytic C-H bond stannylation: a new regioselective pathway to C−Sn bonds via C-H bond functionalization. J. Am. Chem. Soc. 132, 11923–11925 (2010).
Butcher, T. W. et al. Desymmetrization of difluoromethylene groups by C–F bond activation. Nature 583, 548–553 (2020).
Ohashi, M. et al. Palladium-catalyzed coupling reactions of tetrafluoroethylene with arylzinc compounds. J. Am. Chem. Soc. 133, 3256–3259 (2011).
Luo, Y. C., Wang, M. K., Yu, L. C. & Zhang, X. Nickel-catalyzed selective C(sp2)−F bond alkylation of industrially relevant hydrofluoroolefin HFO-1234yf. Angew. Chem. Int. Ed. 62, e202308690 (2023).
Huang, L., Ackerman, L. K. G., Kang, K., Parsons, A. M. & Weix, D. J. LiCl-accelerated multimetallic cross-coupling of aryl chlorides with aryl triflates. J. Am. Chem. Soc. 141, 10978–10983 (2019).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Ackerman, L. K., Lovell, M. M. & Weix, D. J. Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates. Nature 524, 454–457 (2015).
Hamby, T. B., LaLama, M. J. & Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3−Csp2 coupling. Science 376, 410–416 (2022).
Zhang, B. et al. Ni-electrocatalytic Csp3−Csp3 doubly decarboxylative coupling. Nature 606, 313–318 (2022).
Wang, X., Wang, S., Xue, W. & Gong, H.-G. Nickel-catalyzed reductive coupling of aryl bromides with tertiary alkyl halides. J. Am. Chem. Soc. 137, 11562–11565 (2015).
Pang, X., Su, P. & Shu, X.-Z. Reductive Cross-Coupling of Unreactive Electrophiles. Acc. Chem. Res. 55, 2491–2509 (2022).
Cooper, A. K., Leonard, D. K., Bajo, S., Burton, P. M. & Nelson, D. J. Aldehydes and ketones influence reactivity and selectivity in nickel-catalysed Suzuki–Miyaura reactions. Chem. Sci. 11, 1905–1911 (2020).
Li, G. et al. Light-promoted C−N coupling of aryl halides with nitroarenes. Angew. Chem. Int. Ed. 60, 5230–5234 (2021).
Johnson, S. A., Taylor, E. T. & Cruise, S. J. A combined experimental and computational study of unexpected C−F bond activation intermediates and selectivity in the reaction of pentafluorobenzene with a (PEt3)2Ni synthon. Organometallics 28, 3842–3855 (2009).
Sutcliffe, E., Cagan, D. A. & Hadt, R. G. Ultrafast photophysics of Ni(I)–bipyridine halide complexes: spanning the Marcus normal and inverted regimes. J. Am. Chem. Soc. 146, 15506–15514 (2024).
Shields, B. J., Kudisch, B., Scholes, G. D. & Doyle, A. G. Long-lived charge-transfer states of nickel(II) aryl halide complexes facilitate bimolecular photoinduced electron Transfer. J. Am. Chem. Soc. 140, 3035–3039 (2018).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of aryl halides to a Ni(I)-bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
Lin, Q. & Diao, T. Mechanism of Ni-catalyzed reductive 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 17937–17948 (2019).
Ting, S. I. et al. 3d-d Excited states of Ni(II) complexes relevant to photoredox catalysis: spectroscopic identification and mechanistic implications. J. Am. Chem. Soc. 142, 5800–5810 (2020).
Yamamoto, T., Abla, M. & Murakami, Y. Promotion of reductive elimination reaction of diorgano(2,2′-bipyridyl)nickel(II) complexes by electron-accepting aromatic compounds, Lewis acids, and Bronsted acids. Bull. Chem. Soc. Jpn 75, 1997–2009 (2002).
Klein, A., Kaiser, A., Sarkar, B., Wanner, M. & Fiedler, J. The electrochemical behaviour of organonickel complexes: mono-, di- and trivalent nickel. Eur. J. Inorg. Chem. 2007, 965–976 (2007).
Zhang, Z., Niwa, T., Watanabe, K. & Hosoya, T. 11C-Cyanation of aryl fluorides via nickel and lithium chloride-mediated C–F bond activation. Angew. Chem. Int. Ed. 62, e202302956 (2023).
Yuan, M., Song, Z., Badir, S. O., Molander, G. A. & Gutierrez, O. On the nature of C(sp3)–C(sp2) bond formation in nickel-catalyzed tertiary radical cross-couplings: a case study of Ni/photoredox catalytic cross-coupling of alkyl radicals and aryl halides. J. Am. Chem. Soc. 142, 7225–7234 (2020).
Shaik, S., Chen, H. & Janardanan, D. Exchange-enhanced reactivity in bond activation by metal–oxo enzymes and synthetic reagents. Nat. Chem. 3, 19–27 (2011).
Zhang, Y. et al. Catalytic cross-electrophile coupling of aryl chlorides with unactivated alkyl chlorides: the synergy of iron and Li. Chem 9, 3623–3636 (2023).
Liu, C.-X. et al. Explicit mechanism of Rh(I)-catalyzed asymmetric C–H arylation and facile synthesis of planar chiral ferrocenophanes. J. Am. Chem. Soc. 145, 4765–4773 (2023).
Liang, H., Borys, A. M., Hevia, E., Perrin, M.-E. L. & Payard, P.-A. Shedding light on the hidden roles of lithium in the nickel-catalyzed cross-coupling of aryl ethers. J. Am. Chem. Soc. 145, 19989–19999 (2023).
Novak, D. P. & Brown, T. L. Mixed complex formation between methyllithium and lithium bromide or iodide in diethyl ether. J. Am. Chem. Soc. 94, 3793–3798 (1972).
Wang, J.-S., You, X.-X., Zhong, R.-L. & Su, Z.-M. Heterolytic versus homolytic: theoretical insight into the Ni0-catalyzed Ph–F bond activation. Organometallics 42, 2771–2783 (2023).
Luo, Z. J., Zhao, H. Y. & Zhang, X. Highly selective Pd-catalyzed direct C–F bond arylation of polyfluoroarenes. Org. Lett. 20, 2543–2546 (2018).
Wang, X. et al. Ni-catalyzed reductive coupling of electron-rich aryl iodides with tertiary alkyl halides. J. Am. Chem. Soc. 140, 14490–14497 (2018).
Nakao, Y., Kashihara, N., Kanyiva, K. S. & Hiyama, T. Nickel-catalyzed alkenylation and alkylation of fluoroarenes via activation of C−H bond over C–F bond. J. Am. Chem. Soc. 130, 16170–16171 (2008).
Xu, W., Jiang, H., Leng, J., Ong, H. W. & Wu, J. Visible-light-induced selective defluoroborylation of polyfluoroarenes, gem-difluoroalkenes, and trifluoromethylalkenes. Angew. Chem. Int. Ed. 59, 4009–4016 (2020).
Acknowledgements
We thank the National Key Research and Development Program of China (grant no. 2022YFA1503200), National Natural Science Foundation of China (grant nos. 22471121, 22471122 and 22271144) and Fundamental Research Funds for the Central Universities (grant no. 020514380327) for financial support. J. Huang, Y. Cheng and B. Ling are gratefully acknowledged for their reproduction of the experimental procedures for products 16, 32 and 45. All theoretical calculations were performed at the High Performance Computing Center of Nanjing University.
Author information
Authors and Affiliations
Contributions
J.X. and Z.L. conceived the work and designed the experiments. Z.L., K.L., S.X. and W.L. performed the experiments and analysed the experimental data. X.Y. performed the NMR measurements. C.D. and J.H. performed the DFT calculations and discussed the results with C.Z. and J.X. co-wrote the manuscript with input from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Qian Peng, Xingang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1−88, discussion and Tables 1−14.
Supplementary Data 1
Cartesian coordinates of all the optimized geometries.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Du, C., Han, J. et al. Selective arylation of atypical C–F bonds in polyfluoroarenes with aryl chlorides. Nat. Chem. 17, 1655–1665 (2025). https://doi.org/10.1038/s41557-025-01962-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01962-1