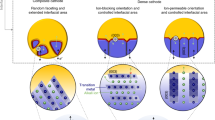
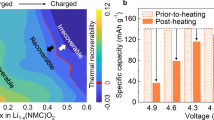
Abstract
The cathode–electrolyte interphase plays a pivotal role in determining the usable capacity and cycling stability of electrochemical cells, yet it is overshadowed by its counterpart, the solid–electrolyte interphase. This is primarily due to the prevalence of side reactions, particularly at low potentials on the negative electrode, especially in state-of-the-art Li-ion batteries where the charge cutoff voltage is limited. However, as the quest for high-energy battery technologies intensifies, there is a pressing need to advance the study of cathode–electrolyte interphase properties. Here, we present a comprehensive approach to analyse the cathode–electrolyte interphase in battery systems. We underscore the importance of employing model cathode materials and coin cell protocols to establish baseline performance. Additionally, we delve into the factors behind the inconsistent and occasionally controversial findings related to the cathode–electrolyte interphase. We also address the challenges and opportunities in characterizing and simulating the cathode–electrolyte interphase, offering potential solutions to enhance its relevance to real-world applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Ohzuku, T., Iwakoshi, Y. & Sawai, K. Formation of lithium–graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 140, 2490 (1993).
Kautz, D. J. et al. Designing electrolytes with controlled solvation structure for fast-charging lithium-ion batteries. Adv. Energy Mater. 13, 2301199 (2023).
Zhang, X. et al. Advanced electrolytes for fast-charging high-voltage lithium-ion batteries in wide-temperature range. Adv. Energy Mater. 10, 2000368 (2020).
Wen, B. et al. Ultrafast ion transport at a cathode–electrolyte interface and its strong dependence on salt solvation. Nat. Energy 5, 578–586 (2020). A study that reveals the relationship between desolvation energy and the CEI’s interfacial kinetics.
Björklund, E. et al. Cycle-induced interfacial degradation and transition-metal cross-over in LiNi0.8Mn0.1Co0.1O2–graphite cells. Chem. Mater. 34, 2034–2048 (2022).
Liu, W. et al. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 11, 3629 (2020).
Zhang, N. et al. Modified cathode–electrolyte interphase toward high-performance batteries. Cell Rep. Phys. Sci. 3, 101197 (2022).
Xu, J. Critical review on cathode–electrolyte interphase toward high-voltage cathodes for Li-ion batteries. Nano-Micro Lett. 14, 166 (2022).
Gutierrez, A. et al. Review—Earth-abundant, Mn-rich cathodes for vehicle applications and beyond: overview of critical barriers. J. Electrochem. Soc. 170, 030509 (2023).
Clément, R. J., Lun, Z. & Ceder, G. Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes. Energy Environ. Sci. 13, 345–373 (2020).
Nelson, K. J. et al. Studies of the effect of high voltage on the impedance and cycling performance of Li[Ni0.4Mn0.4Co0.2]O2/graphite lithium-ion pouch cells. J. Electrochem. Soc. 162, A1046–A1054 (2015).
Yan, P. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 3, 600–605 (2018).
Hu, J. et al. Locking oxygen in lattice: a quantifiable comparison of gas generation in polycrystalline and single crystal Ni-rich cathodes. Energy Storage Mater. 47, 195–202 (2022).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020). A study on how to make a single-crystal, nickel-rich cathode more robust and more efficient.
Lin, F. et al. Metal segregation in hierarchically structured cathode materials for high-energy lithium batteries. Nat. Energy 1, 15004 (2016).
Yan, P. et al. Ni and Co segregations on selective surface facets and rational design of layered lithium transition-metal oxide cathodes. Adv. Energy Mater. 6, 1502455 (2016).
Zhang, J. et al. Interfacial design for a 4.6 V high-voltage single-crystalline LiCoO2 cathode. Adv. Mater. 34, 2108353 (2022).
Choi, S. H., Son, J.-W., Yoon, Y. S. & Kim, J. Particle size effects on temperature-dependent performance of LiCoO2 in lithium batteries. J. Power Sources 158, 1419–1424 (2006).
Zhang, X. et al. Electrolyte regulating toward stabilization of cobalt-free ultrahigh-nickel layered oxide cathode in lithium-ion batteries. ACS Energy Lett. 6, 1324–1332 (2021).
Jung, R. et al. Effect of ambient storage on the degradation of Ni-rich positive electrode materials (NMC811) for Li-ion batteries. J. Electrochem. Soc. 165, A132–A141 (2018).
Liu, D. et al. Review of recent development of in situ/operando characterization techniques for lithium battery research. Adv. Mater. 31, 1806620 (2019).
Borkiewicz, O. J., Wiaderek, K. M., Chupas, P. J. & Chapman, K. W. Best practices for operando battery experiments: influences of X-ray experiment design on observed electrochemical reactivity. J. Phys. Chem. Lett. 6, 2081–2085 (2015).
Chen, S. et al. Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries. Joule 3, 1094–1105 (2019). A study that proposed coin cell and pouch cell critical parameters and testing conditions for the battery research community to bridge the gap between fundamental research and practical adoption of new ideas or materials and to expedite their full integration into realistic battery systems.
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Xiao, J. et al. Perspective—Electrochemistry in understanding and designing electrochemical energy storage systems. J. Electrochem. Soc. 169, 010524 (2022).
Hu, J. et al. Achieving highly reproducible results in graphite-based Li-ion full coin cells. Joule 5, 1011–1015 (2021).
Bi, Y. et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure. Energy Storage Mater. 24, 291–296 (2020).
Lu, D. et al. Enabling high-energy-density cathode for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 10, 23094–23102 (2018).
Xiao, J. Understanding the lithium sulfur battery system at relevant scales. Adv. Energy Mater. 5, 1501102 (2015).
Zhang, N. et al. Long-term cycling and mechanisms of cell degradation of single crystal LiNi0.95Mn0.04Co0.01O2/graphite cells. J. Electrochem. Soc. 171, 010520 (2024).
Bi, Y., Li, Q., Yi, R. & Xiao, J. To pave the way for large-scale electrode processing of moisture-sensitive Ni-rich cathodes. J. Electrochem. Soc. 169, 020521 (2022).
Cao, X. Important factors for the reliable and reproducible preparation of non-aqueous electrolyte solutions for lithium batteries. Commun. Mater. 4, 10 (2023).
Klein, S. et al. Suppressing electrode crosstalk and prolonging cycle life in high-voltage Li ion batteries: pivotal role of fluorophosphates in electrolytes. ChemElectroChem 9, e202200469 (2022).
Zhang, X., Cui, Z. & Manthiram, A. Insights into the crossover effects in cells with high-nickel layered oxide cathodes and silicon/graphite composite anodes. Adv. Energy Mater. 12, 2103611 (2022).
Wu, Q., McDowell, M. T. & Qi, Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries. J. Am. Chem. Soc. 145, 2473–2484 (2023).
Cao, X. et al. Optimization of fluorinated orthoformate based electrolytes for practical high-voltage lithium metal batteries. Energy Storage Mater. 34, 76–84 (2021).
Yamada, Y. et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 136, 5039–5046 (2014).
Peljo, P. & Girault, H. H. Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception. Energy Environ. Sci. 11, 2306–2309 (2018).
Dong, T. et al. Electrolyte engineering toward high performance high nickel (Ni ≥ 80%) lithium-ion batteries. Adv. Sci. 11, 2305753 (2023).
Rinkel, B. L. D., Vivek, J. P., Garcia-Araez, N. & Grey, C. P. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries. Energy Environ. Sci. 15, 3416–3438 (2022).
Wu, Y. et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte. Adv. Energy Mater. 11, 2102299 (2021).
Dai, P. et al. Synergistic effect of dual-anion additives promotes the fast dynamics and high-voltage performance of Ni-rich lithium-ion batteries by regulating the electrode/electrolyte interface. ACS Appl. Mater. Interfaces 14, 39927–39938 (2022).
Zhang, J. et al. Multifunctional solvent molecule design enables high-voltage Li-ion batteries. Nat. Commun. 14, 2211 (2023).
Lu, D. et al. A self-purifying electrolyte enables high energy Li ion batteries. Energy Environ. Sci. 15, 3331–3342 (2022).
Zou, Y. et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging. Angew. Chem. Int. Ed. 62, e202216189 (2023).
An, K., Tran, Y. H. T., Kwak, S., Han, J. & Song, S.-W. Design of fire-resistant liquid electrolyte formulation for safe and long-cycled lithium-ion batteries. Adv. Funct. Mater. 31, 2106102 (2021).
Wu, C. et al. Thermal runaway suppression of high-energy lithium-ion batteries by designing the stable interphase. J. Electrochem. Soc. 168, 090563 (2021).
Hou, J. et al. Thermal runaway of lithium-ion batteries employing flame-retardant fluorinated electrolytes. Energy Environ. Mater. 6, e12297 (2023).
Chen, L. et al. High-safety and high-efficiency electrolyte design for 4.6 V-class lithium-ion batteries with a non-solvating flame-retardant. Chem. Sci. 14, 1184–1193 (2023).
Fang, M. et al. An electrolyte with less space-occupying diluent at cathode inner Helmholtz plane for stable 4.6 V lithium-ion batteries. Angew. Chem. Int. Ed. 63, e202316839 (2023).
Xu, J. et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 614, 694–700 (2023). A work that reports an electrolyte design principle to form stable SEI/CEI for high-energy batteries operating under extreme conditions.
Jia, H. et al. Toward the practical use of cobalt-free lithium-ion batteries by an advanced ether-based electrolyte. ACS Appl. Mater. Interfaces 13, 44339–44347 (2021).
Páez Fajardo, G. J. et al. Synergistic degradation mechanism in single crystal Ni-rich NMC//graphite cells. ACS Energy Lett. 8, 5025–5031 (2023).
Li, J., Downie, L. E., Ma, L., Qiu, W. & Dahn, J. R. Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for lithium ion batteries. J. Electrochem. Soc. 162, A1401–A1408 (2015).
Jo, M., Park, S.-H. & Lee, H. NaH2PO4 as an electrolyte additive for enhanced thermal stability of LiNi0.8Co0.1Mn0.1O2/graphite batteries. J. Electrochem. Soc. 167, 130502 (2020).
Li, J., Li, W., You, Y. & Manthiram, A. Extending the service life of high-Ni layered oxides by tuning the electrode–electrolyte interphase. Adv. Energy Mater. 8, 1801957 (2018).
Li, S. et al. 3,3-diethylene di-sulfite (DES) as a high-voltage electrolyte additive for 4.5 V LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performances. ChemElectroChem 8, 745–754 (2021).
Ren, Z. et al. Delicately designed cyano-siloxane as multifunctional additive enabling high voltage LiNi0.9Co0.05Mn0.05O2/graphite full cell with long cycle life at 50 °C. Adv. Funct. Mater. 33, 2302411 (2023).
Zhao, W. et al. Extending the high-voltage operation of graphite/NCM811 cells by constructing a robust electrode/electrolyte interphase layer. Mater. Today Energy 34, 101301 (2023).
Cheng, F. et al. Tailoring electrolyte enables high-voltage Ni-rich NCM cathode against aggressive cathode chemistries for Li-ion batteries. Sci. Bull. 67, 2225–2234 (2022).
Ma, L., Xia, J. & Dahn, J. R. Improving the high voltage cycling of Li[Ni0.42Mn0.42Co0.16]O2 (NMC442)/graphite pouch cells using electrolyte additives. J. Electrochem. Soc. 161, A2250–A2254 (2014).
Xia, J. et al. Study of triallyl phosphate as an electrolyte additive for high voltage lithium-ion cells. J. Power Sources 295, 203–211 (2015).
Keefe, A. S., Weber, R., Hill, I. G. & Dahn, J. R. Studies of the SEI layers in Li(Ni0.5Mn0.3Co0.2)O2/artificial graphite cells after formation and after cycling. J. Electrochem. Soc. 167, 120507 (2020).
Thompson, L. M. et al. Quantifying changes to the electrolyte and negative electrode in aged NMC532/graphite lithium-ion cells. J. Electrochem. Soc. 165, A2732–A2740 (2018).
Thomas, M. G. S. R., Bruce, P. G. & Goodenough, J. B. AC impedance analysis of polycrystalline insertion electrodes—application to Li1−xCoO2. J. Electrochem. Soc. 132, 1521–1528 (1985).
Demeaux, J., Caillon-Caravanier, M., Galiano, H., Lemordant, D. & Claude-Montigny, B. LiNiMnO/electrolyte and carbon black/electrolyte high voltage interfaces: to evidence the chemical and electronic contributions of the solvent on the cathode–electrolyte interface formation. J. Electrochem. Soc. 159, A1880–A1890 (2012).
Gauthier, M. et al. Electrode–electrolyte interface in Li-ion batteries: current understanding and new insights. J. Phys. Chem. Lett. 6, 4653–4672 (2015).
Zhang, Z. W. et al. Cathode–electrolyte interphase in lithium batteries revealed by cryogenic electron microscopy. Matter 4, 302–312 (2021).
Huang, W., Wang, H., Boyle, D. T., Li, Y. Z. & Cui, Y. Resolving nanoscopic and mesoscopic heterogeneity of fluorinated species in battery solid–electrolyte interphases by cryogenic electron microscopy. ACS Energy Lett. 5, 1128–1135 (2020).
Hestenes, J. C. & Marbella, L. E. Beyond composition: surface reactivity and structural arrangement of the cathode–electrolyte interphase. ACS Energy Lett. 8, 4572–4596 (2023).
Lu, J., Wu, T. P. & Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2, 17011 (2017). A review that summarizes the application of advanced characterization techniques for lithium-ion batteries.
Xu, Y. et al. Direct in situ measurements of electrical properties of solid–electrolyte interphase on lithium metal anodes. Nat. Energy 8, 1345–1354 (2023).
Xiao, J., Shi, F. F., Glossmann, T., Burnett, C. & Liu, Z. From laboratory innovations to materials manufacturing for lithium-based batteries. Nat. Energy 8, 329–339 (2023). A Perspective, which reviews challenges and opportunities in scaling up lithium-based battery materials and components to accelerate future low-cost battery manufacturing.
Huang, J. Q., Boles, S. T. & Tarascon, J. M. Sensing as the key to battery lifetime and sustainability. Nat. Sustain. 5, 194–204 (2022).
Yao, N., Chen, X., Fu, Z. & Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 122, 10970–11021 (2022).
Tan, X. et al. Decoding electrochemical processes of lithium-ion batteries by classical molecular dynamics simulations. Adv. Energy Mater. 14, 2400564 (2024).
Kim, S., van Duin, A. C. T. & Shenoy, V. B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: a molecular dynamics study. J. Power Sources 196, 8590–8597 (2011).
Alzate-Vargas, L. et al. Insight into SEI growth in Li-ion batteries using molecular dynamics and accelerated chemical reactions. J. Phys. Chem. C 125, 18588–18596 (2021).
Gao, H. et al. Enhanced electrolyte transport and kinetics mitigate graphite exfoliation and Li plating in fast-charging Li-ion batteries. Adv. Energy Mater. 13, 2202906 (2023).
Holoubek, J. et al. Predicting the ion desolvation pathway of lithium electrolytes and their dependence on chemistry and temperature. J. Phys. Chem. Lett. 13, 4426–4433 (2022).
Diddens, D. et al. Modeling the solid electrolyte interphase: machine learning as a game changer? Adv. Mater. Interfaces 9, 2101734 (2022). A review that summarizes recent advances in the integration and application of physics-based modeling and ML to the study of solid electrolyte interphases in lithium-ion batteries.
Shao, Y., Knijff, L., Dietrich, F. M., Hermansson, K. & Zhang, C. Modelling bulk electrolytes and electrolyte interfaces with atomistic machine learning. Batter. Supercaps 4, 585–595 (2021).
Saritas, K., Fadel, E. R., Kozinsky, B. & Grossman, J. C. Charge density and redox potential of LiNiO2 using ab initio diffusion quantum Monte Carlo. J. Phys. Chem. C 124, 5893–5901 (2020).
Timrov, I., Aquilante, F., Cococcioni, M. & Marzari, N. Accurate electronic properties and intercalation voltages of olivine-type Li-ion cathode materials from extended Hubbard functionals. PRX Energy 1, 033003 (2022).
Sun, W. et al. Coupled experimental–theoretical characterization of a carbon electrode in vanadium redox flow batteries using X-ray absorption spectroscopy. ACS Appl. Mater. Interfaces 16, 8791–8801 (2024). A study that demonstrates tight experiment–theory integration for resolving the impact of electrode surface chemistry on electrochemical cell performance.
Tamura, T., Kohyama, M. & Ogata, S. Combination of first-principles molecular dynamics and XANES simulations for LiCoO2–electrolyte interfacial reactions in a lithium-ion battery. Phys. Rev. B 96, 035107 (2017).
Carlier, D. et al. DFT + U calculations and XAS study: further confirmation of the presence of CoO5 square-based pyramids with IS-Co3+ in Li-overstoichiometric LiCoO2. J. Phys. Chem. C 117, 26493–26500 (2013).
Xiao, J. Cathode–Electrolyte Interphase (CEI) Consortium: Model Cathode Materials for Next-Generation Li-Ion Batteries Annual merit review meeting (US Department of Energy Vehicle Technology Office, 2024).
Bi, Y. et al. Simultaneous single crystal growth and segregation of Ni-rich cathode enabled by nanoscale phase separation for advanced lithium-ion batteries. Energy Storage Mater. 62, 102947 (2023).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) through the Cathode–Electrolyte Interphase (CEI) Consortium. Pacific Northwest National Laboratory (PNNL) is operated by Battelle for the DOE under contract DE-AC05-76RL01830. Part of the work was performed under the auspices of the US DOE by Lawrence Livermore National Laboratory under contract DEAC52-07NA27344. Sandia National Laboratories is a multi-mission laboratory managed and operated by National Technology and Engineering Solutions of Sandia LLC, a wholly-owned subsidiary of Honeywell International Inc., for the US DOE’s National Nuclear Security Administration under contract DE-NA-0003525.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yang-Kook Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Adelstein, N., Bi, Y. et al. Assessing cathode–electrolyte interphases in batteries. Nat Energy 9, 1463–1473 (2024). https://doi.org/10.1038/s41560-024-01639-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41560-024-01639-y
This article is cited by
-
Unusual Li2O sublimation promotes single-crystal growth and sintering
Nature Energy (2025)
-
Battery researchers strive for standardization
Nature (2025)