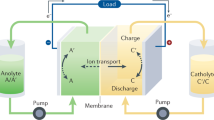
Abstract
The economic viability of flow battery systems has garnered substantial attention in recent years, but technoeconomic models often overlook the costs associated with electrolyte tanks. This work challenges the commonly assumed insignificance of electrolyte tank costs in flow battery research and demonstrates their substantial impact on overall system economics. Using prices quoted by globally distributed tank manufacturers, it is shown that tank costs in most published technoeconomic models are severely underestimated, if not entirely neglected. Back-of-the-envelope calculations show that electrolyte tanks may constitute up to 40% of the energy component (tank plus electrolyte) costs in MWh-scale flow battery systems. Standardization of flow battery components and the development of high-voltage chemistries are highlighted as paths towards decreasing costs and achieving greater market penetration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sánchez-Díez, E. et al. Redox flow batteries: status and perspective towards sustainable stationary energy storage. J. Power Sources 481, 228804 (2021).
Zhu, Z. et al. Rechargeable batteries for grid scale energy storage. Chem. Rev. 122, 16610–16751 (2022).
Huang, Z. et al. Comprehensive analysis of critical issues in all-vanadium redox flow battery. ACS Sustain. Chem. Eng. 10, 7786–7810 (2022).
Li, Z. & Lu, Y. C. Material design of aqueous redox flow batteries: fundamental challenges and mitigation strategies. Adv. Mater. 32, 2002132 (2020).
Albertus, P., Manser, J. S. & Litzelman, S. Long-duration electricity storage applications, economics and technologies. Joule 4, 21–32 (2020).
Li, Z. et al. Air-breathing aqueous sulfur flow battery for ultralow-cost long-duration electrical storage. Joule 1, 306–327 (2017).
Brushett, F. R., Aziz, M. J. & Rodby, K. E. On lifetime and cost of redox-active organics for aqueous flow batteries. ACS Energy Lett. 5, 879–884 (2020).
Reber, D., Jarvis, S. R. & Marshak, M. P. Beyond energy density: flow battery design driven by safety and location. Energy Adv. 2, 1357–1365 (2023).
Viva energy opens Australia’s largest crude oil tank at the Geelong Refinery. Viva Energy Australia (22 November 2017); https://www.vivaenergy.com.au/media/news/2017/viva-energy-opens-australias-largest-crude-oil-tank-at-the-geelong-refinery
Reber, D., Thurston, J. R., Becker, M. & Marshak, M. P. Stability of highly soluble ferrocyanides at neutral pH for energy-dense flow batteries. Cell Rep. Phys. Sci. 4, 101215 (2023).
Trovò, A., Prieto-Díaz, P. A., Zatta, N., Picano, F. & Guarnieri, M. Early investigations on electrolyte mixing issues in large flow battery tanks. Batteries 10, 133 (2024).
Wang, H., Pourmousavi, S. A., Soong, W. L., Zhang, X. & Ertugrul, N. Battery and energy management system for vanadium redox flow battery: a critical review and recommendations. J. Energy Storage 58, 106384 (2023).
Prieto-Díaz, P. A., Ibáñez, S. E. & Vera, M. Fluid dynamics of mixing in the tanks of small vanadium redox flow batteries: insights from order-of-magnitude estimates and transient two-dimensional simulations. Int. J. Heat Mass Transf. 216, 124567 (2023).
Horne, C. R., Hickey, D. B., Kinoshita, K., Mosso, R. J. & Lin, B. Redox flow battery system with divided tank system. PCT patent US20130011702A1 (2013).
Liu, B., Zheng, M., Sun, J. & Yu, Z. No-mixing design of vanadium redox flow battery for enhanced effective energy capacity. J. Energy Storage 23, 278–291 (2019).
Nemani, V. P. & Smith, K. C. Uncovering the role of flow rate in redox-active polymer flow batteries: simulation of reaction distributions with simultaneous mixing in tanks. Electrochim. Acta 247, 475–485 (2017).
Mengenschwellen Gemäss Störfallverordnung (StFV) (Bundesamt für Umwelt BAFU, 2024); https://www.bafu.admin.ch/bafu/de/home/themen/stoerfallvorsorge/publikationen-studien/publikationen/mengenschwellen-gemaess-stoerfallverordnung.html
Minke, C. & Ledesma, M. A. D. Impact of cell design and maintenance strategy on life cycle costs of vanadium redox flow batteries. J. Energy Storage 21, 571–580 (2019).
Minke, C., Kunz, U. & Turek, T. Techno-economic assessment of novel vanadium redox flow batteries with large-area cells. J. Power Sources 361, 105–114 (2017).
Poli, N., Bonaldo, C., Moretto, M. & Guarnieri, M. Techno-economic assessment of future vanadium flow batteries based on real device/market parameters. Appl. Energy 362, 122954 (2024).
Milshtein, J. D., Darling, R. M., Drake, J., Perry, M. L. & Brushett, F. R. The critical role of supporting electrolyte selection on flow battery cost. J. Electrochem. Soc. 164, A3883 (2017).
Viswanathan, V. et al. Cost and performance model for redox flow batteries. J. Power Sources 247, 1040–1051 (2014).
Rodby, K. E., Jaffe, R. L., Olivetti, E. A. & Brushett, F. R. Materials availability and supply chain considerations for vanadium in grid-scale redox flow batteries. J. Power Sources 560, 232605 (2023).
Darling, R. M. Techno-economic analyses of several redox flow batteries using levelized cost of energy storage. Curr. Opin. Chem. Eng. 37, 100855 (2022).
Dmello, R., Milshtein, J. D., Brushett, F. R. & Smith, K. C. Cost-driven materials selection criteria for redox flow battery electrolytes. J. Power Sources 330, 261–272 (2016).
Ha, S. & Gallagher, K. G. Estimating the system price of redox flow batteries for grid storage. J. Power Sources 296, 122–132 (2015).
Darling, R. M., Gallagher, K. G., Kowalski, J. A., Ha, S. & Brushett, F. R. Pathways to low-cost electrochemical energy storage: a comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 7, 3459–3477 (2014).
Turker, B., Klein, S. A., Hammer, E.-M., Lenz, B. & Komsiyska, L. Modeling a vanadium redox flow battery system for large scale applications. Energy Convers. Manag. 66, 26–32 (2013).
Li, Y., Kienbaum, D., Lüth, T. & Skyllas-Kazacos, M. Long term performance evaluation of a commercial vanadium flow battery system. J. Energy Storage 90, 111790 (2024).
Noack, J., Wietschel, L., Roznyatovskaya, N., Pinkwart, K. & Tübke, J. Techno-economic modeling and analysis of redox flow battery systems. Energies 9, 627 (2016).
ISO Tank Container (Henan Lishixin Logistics Equipment Co. Ltd, 2024); https://hnlsxtruck.en.made-in-china.com/product/pdRfMWaoYVcE/China-25000-to-26000-Litre-Un-Portable-T11-20FT-Liquid-ISO-Tank-Container-for-Sale.html
Arjun Bhattarai VFlowTech Pte Ltd. Performance Evaluation of a 400 kW-1600 kWh System Designed for Oil Terminals (International Flow Battery Forum, 2024).
Kusano, M., Kanai, T., Arao, Y. & Kubouchi, M. Degradation behavior and lifetime estimation of fiber reinforced plastics tanks for hydrochloric acid storage. Eng. Fail. Anal. 79, 971–979 (2017).
Amaro, A., Reis, P., Neto, M. & Louro, C. Effects of alkaline and acid solutions on glass/epoxy composites. Polym. Degrad. Stab. 98, 853–862 (2013).
Tang, L. et al. Capital cost evaluation of conventional and emerging redox flow batteries for grid storage applications. Electrochim. Acta 437, 141460 (2023).
Amini, K., Shocron, A. N., Suss, M. E. & Aziz, M. J. Pathways to high-power-density redox flow batteries. ACS Energy Lett. 8, 3526–3535 (2023).
Robb, B. H., Waters, S. E., Saraidaridis, J. D. & Marshak, M. P. Realized potential as neutral pH flow batteries achieve high power densities. Cell Rep. Phys. Sci 3, 101118 (2022).
Perry, M. L., Rodby, K. E. & Brushett, F. R. Untapped potential: the need and opportunity for high-voltage aqueous redox flow batteries. ACS Energy Lett. 7, 659–667 (2022).
Suo, L. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Yamada, Y. et al. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 1, 16129 (2016).
Reber, D., Figi, R., Kuehnel, R.-S. & Battaglia, C. Stability of aqueous electrolytes based on LiFSI and NaFSI. Electrochim. Acta 321, 134644 (2019).
Ding, M. S., von Cresce, A. & Xu, K. Conductivity, viscosity and their correlation of a super-concentrated aqueous electrolyte. J. Phys. Chem. C 121, 2149–2153 (2017).
Amini, K., Gostick, J. & Pritzker, M. D. Metal and metal oxide electrocatalysts for redox flow batteries. Adv. Funct. Mater. 30, 1910564 (2020).
Jiang, Q. et al. Recent advances in carbon-based electrocatalysts for vanadium redox flow battery: mechanisms, properties and perspectives. Compos. B. Eng. 242, 110094 (2022).
Proctor, A. D., Robb, B. H., Saraidaridis, J. D. & Marshak, M. P. Bismuth electrocatalyst enabling reversible redox kinetics of a chelated chromium flow battery anolyte. J. Electrochem. Soc. 169, 030506 (2022).
Jacquemond, R. R. et al. Microstructural engineering of high-power redox flow battery electrodes via non-solvent induced phase separation. Cell Rep. Phys. Sci. 3, 100943 (2022).
van Gorp, R., van der Heijden, M., Sadeghi, M. A., Gostick, J. & Forner-Cuenca, A. Bottom-up design of porous electrodes by combining a genetic algorithm and a pore network model. Chem. Eng. J. 455, 139947 (2023).
van der Heijden, M., Kroese, M., Borneman, Z. & Forner‐Cuenca, A. Investigating mass transfer relationships in stereolithography 3D printed electrodes for redox flow batteries. Adv. Mater. Technol. 8, 2300611 (2023).
Xi, D. et al. Mild pH-decoupling aqueous flow battery with practical pH recovery. Nat. Energy 9, 479–490 (2024).
Jin, S. et al. Near neutral pH redox flow battery with low permeability and long‐lifetime phosphonated viologen active species. Adv. Energy Mater. 10, 2000100 (2020).
Zhang, C., Yuan, Z. & Li, X. Designing better flow batteries: an overview on fifty years’ research. ACS Energy Lett. 9, 3456–3473 (2024).
Luo, J. et al. A 1.51 V pH neutral redox flow battery towards scalable energy storage. J. Mater. Chem. A 7, 9130–9136 (2019).
Peng, K. et al. Progress and prospects of pH-neutral aqueous organic redox flow batteries: electrolytes and membranes. J. Energy Chem. 96, 89–109 (2024).
Acknowledgements
D.R. acknowledges funding from the Swiss National Science Foundation (SNSF) Ambizione Fellowship Z00P2_209078. D.R. also thanks Corsin Battaglia and Empa’s Laboratory Materials for Energy Conversion for hosting his research group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Energy thanks Massimo Guarnieri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source Data Fig. 1
Quoted prices shown in Fig. 1a
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reber, D. Electrolyte tank costs are an overlooked factor in flow battery economics. Nat Energy 10, 23–27 (2025). https://doi.org/10.1038/s41560-024-01677-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-024-01677-6
This article is cited by
-
Water dissociation efficiencies control the viability of reverse-bias bipolar membranes for CO2 electrolysis
Nature Chemical Engineering (2025)