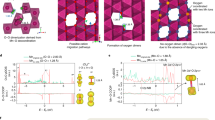
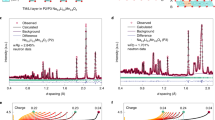
Abstract
Oxide cathodes enable high-energy lithium-ion and sodium-ion batteries, with their performances fundamentally governed by three interrelated chemical factors: electronic configuration, chemical bonding and chemical reactivity. Here we illustrate how these factors dictate the redox energy, structural stability, ionic and electronic transport, and interfacial behaviour in both layered oxide and polyanion oxide cathodes. We discuss how crystal field effects and octahedral-site stabilization energies influence cation migration, and how inductive effects tune bond covalency and operating voltages. We also explain how chemical bonding governs thermal stability, gas evolution and first-cycle capacity loss, and how alignment of the transition metal redox band with the oxygen 2p band determines electrolyte reactivity. A comparison between lithium and sodium layered oxides further reveals how differences in Li–O and Na–O bond ionicity affect chemical reactivity. Finally, we outline strategies, including compositional tuning, surface doping and electrolyte optimization, for the development of new materials with improved performance, and emphasize how high-throughput, data-driven approaches can offer guidance for the design of next-generation oxide cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Battery market size, share, and trends 2025 to 2034. Precedence Research https://www.precedenceresearch.com/battery-market (2025).
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Mizushima, K., Jones, P. C., Wiseman, P. J. & Goodenough, J. B. LixCoO2 (0 < x < −1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
Thackeray, M. M., David, W. I. F., Bruce, P. G. & Goodenough, J. B. Lithium insertion into manganese spinels. Mater. Res. Bull. 18, 461–472 (1983).
Manthiram, A. & Goodenough, J. B. Lithium insertion into Fe2(MO4)3 frameworks: comparison of M = W with M = Mo. J. Solid State Chem. 71, 349–360 (1987).
Manthiram, A. & Goodenough, J. B. Lithium insertion into Fe2(SO4)3 frameworks. J. Power Sources 26, 403–408 (1989).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
Kwade, A. et al. Current status and challenges for automotive battery production technologies. Nat. Energy 3, 290–300 (2018).
Li, W., Erickson, E. M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Armstrong, A. R., Dupre, N., Paterson, A. J., Grey, C. P. & Bruce, P. G. Combined neutron diffraction, NMR, and electrochemical investigation of the layered-to-spinel transformation in LiMnO2. Chem. Mater. 16, 3106–3118 (2004).
Dunitz, J. D. & Orgel, L. E. Electronic properties of transition-metal oxides-II. J. Phys. Chem. Solids 3, 318–323 (1957).
McClure, D. S. The distribution of transition metal cations in spinels. J. Phys. Chem. Solids 3, 311–317 (1957).
Zhan, C. et al. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 4, 2437 (2013).
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022).
Wang, P.-F., You, Y., Yin, Y.-X. & Guo, Y.-G. Layered oxide cathodes for sodium-ion batteries: phase transition, air stability, and performance. Adv. Energy Mater. 8, 1701912 (2018).
Guo, Y.-J. et al. Sodium layered oxide cathodes: properties, practicality and prospects. Chem. Soc. Rev. 53, 7828–7874 (2024).
Asl, H. Y. & Manthiram, A. Reining in dissolved transition-metal ions. Science 369, 140–141 (2020).
Asl, H. Y. & Manthiram, A. Proton-induced disproportionation of Jahn–Teller-active transition-metal ions in oxides due to electronically driven lattice instability. J. Am. Chem. Soc. 142, 21122–21130 (2020).
Chebiam, R. V., Kannan, A. M., Prado, F. & Manthiram, A. Comparison of the chemical stability of the high energy density cathodes of lithium-ion batteries. Electrochem. Commun. 3, 624–627 (2001).
Nishizawa, M. & Yamamura, S. Irreversible conductivity change of Li1−xCoO2 on electrochemical lithium insertion/extraction, desirable for battery applications. Chem. Commun. 1631–1632 (1998).
Chen, L., Cui, Z. & Manthiram, A. Decoding gas evolution pathways and interfacial chemistry in layered oxide cathodes for safer sodium-ion batteries. Adv. Energy Mater. https://doi.org/10.1002/aenm.202504756 (2025).
Cui, Z., Liu, C., Wang, F. & Manthiram, A. Navigating thermal stability intricacies of high-nickel cathodes for high-energy lithium batteries. Nat. Energy 10, 490–501 (2025).
Guo, Z., Cui, Z. & Manthiram, A. Reducing the initial capacity loss in high-nickel cathodes with a higher upper cut-off voltage formation cycle protocol. ACS Energy Lett. 9, 3316–3323 (2024).
Croguennec, L., Pouillerie, C. & Delmas, C. Structural characterisation of new metastable NiO2 phases. Solid State Ion. 135, 259–266 (2000).
Pouillerie, C., Croguennec, L. & Delmas, C. The LixNi1−yMgyO2 (y = 0.05, 0.10) system: structural modifications observed upon cycling. Solid State Ion. 132, 15–29 (2000).
Croguennec, L., Pouillerie, C. & Delmas, C. NiO2 obtained by electrochemical lithium deintercalation from lithium nickelate: structural modifications. J. Electrochem. Soc. 147, 1314–1321 (2000).
Cui, Z., Guo, Z. & Manthiram, A. Assessing the intrinsic roles of key dopant elements in high-nickel layered oxide cathodes in lithium-based batteries. Adv. Energy Mater. 13, 2203853 (2023).
Choi, J. & Manthiram, A. Structural and electrochemical characterization of the layered LiNi0.5−yMn0.5−yCo2yO2 (0 ≤ 2y ≤ 1) cathodes. Solid State Ion. 176, 2251–2256 (2005).
Cho, J., Kim, Y. J. & Park, B. Novel LiCoO2 cathode material with Al2O3 coating for a Li ion cell. Chem. Mater. 12, 3788–3791 (2000).
Liu, Q. et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 3, 936–943 (2018).
Yamada, S., Fujiwara, M. & Kanda, M. Synthesis and properties of LiNiO2 as cathode material for secondary batteries. J. Power Sources 54, 209–213 (1995).
Li, H., Zhang, N., Li, J. & Dahn, J. R. Updating the structure and electrochemistry of LixNiO2 for 0 ≤ x ≤ 1. J. Electrochem. Soc. 165, A2985–A2993 (2018).
Jung, R., Metzger, M., Maglia, F., Stinner, C. & Gasteiger, H. A. Chemical versus electrochemical electrolyte oxidation on NMC111, NMC622, NMC811, LNMO, and conductive carbon. J. Phys. Chem. Lett. 8, 4820–4825 (2017).
Wandt, J., Freiberg, A. T. S., Ogrodnik, A. & Gasteiger, H. A. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 21, 825–833 (2018).
Jung, R., Strobl, P., Maglia, F., Stinner, C. & Gasteiger, H. A. Temperature dependence of oxygen release from LiNi0.6Mn0.2Co0.2O2 (NMC622) cathode materials for Li-ion batteries. J. Electrochem. Soc. 165, A2869–A2879 (2018).
Sim, R., Cui, Z. & Manthiram, A. Impact of dopants on suppressing gas evolution from high-nickel layered oxide cathodes. ACS Energy Lett. 8, 5143–5148 (2023).
Sim, R. & Manthiram, A. Factors influencing gas evolution from high-nickel layered oxide cathodes in lithium-based batteries. Adv. Energy Mater. 14, 2303985 (2024).
Cui, Z., Zuo, P., Guo, Z., Wang, C. & Manthiram, A. Formation and detriments of residual alkaline compounds on high-nickel layered oxide cathodes. Adv. Mater. 36, 2402420 (2024).
Liu, C., Cui, Z. & Manthiram, A. Tuning dopant distribution for stabilizing the surface of high-nickel layered oxide cathodes for lithium-ion batteries. Adv. Energy Mater. 14, 2302722 (2024).
Pan, R., Jo, E., Cui, Z. & Manthiram, A. Degradation pathways of cobalt-free LiNiO2 cathode in lithium batteries. Adv. Funct. Mater. 33, 2211461 (2023).
Lee, S., Su, L., Mesnier, A., Cui, Z. & Manthiram, A. Cracking vs. surface reactivity in high-nickel cathodes for lithium-ion batteries. Joule 7, 2430–2444 (2023).
Nisar, U., Muralidharan, N., Essehli, R., Amin, R. & Belharouak, I. Valuation of surface coatings in high-energy density lithium-ion battery cathode materials. Energy Storage Mater. 38, 309–328 (2021).
Wang, L. et al. Unravelling the origin of irreversible capacity loss in NaNiO2 for high voltage sodium ion batteries. Nano Energy 34, 215–223 (2017).
Sada, K., Kmiec, S. & Manthiram, A. Mitigating sodium ordering for enhanced solid solution behavior in layered NaNiO2 cathodes. Angew. Chem. Int. Ed. 63, e202403865 (2024).
Zuo, W. et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres. Nat. Commun. 11, 3544 (2020).
Zuo, W. et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries. Nat. Commun. 12, 4903 (2021).
Yang, Y. et al. Decoupling the air sensitivity of Na-layered oxides. Science 385, 744–752 (2024).
Ye, Z. et al. Impact of salts and linear carbonates on the performance of layered oxide/hard carbon sodium-ion pouch cells with alkyl carbonate electrolytes. J. Electrochem. Soc. 171, 040522 (2024).
Hijazi, H. et al. Can layered oxide/hard carbon sodium-ion pouch cells with simple electrolyte additives achieve better cycle life than LFP/graphite cells? J. Electrochem. Soc. 171, 050521 (2024).
Acknowledgements
This work was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under award number DE-SC0005397 and the Assistant Secretary for Energy Efficiency and Renewable Energy (EERE), Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) award number DE-AC05-76RLO1830.
Author information
Authors and Affiliations
Contributions
A.M. and Z.C. conceived the idea and wrote the paper. A.M. supervised the work.
Corresponding author
Ethics declarations
Competing interests
A.M. is a co-founder of TexPower EV Technologies, a company focusing on cobalt-free cathode materials for lithium-based batteries. Z.C. declares no competing interests.
Peer review
Peer review information
Nature Energy thanks Gui-Liang Xu, Lin Gu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manthiram, A., Cui, Z. Chemical factors controlling the behaviour of oxide cathodes in batteries. Nat Energy (2026). https://doi.org/10.1038/s41560-025-01963-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41560-025-01963-x