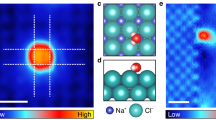
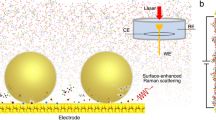
Abstract
The interaction modes of water with (polar) solids are manifold, comprising surface adsorption and incorporation into the bulk, both in molecular and in dissociated form. This Review discusses these processes and the respective pronounced effects on the ionic transport properties. The concentration as well as the mobility of ionic carriers can vary by orders of magnitude depending on the water content on or within a solid. Selected materials examples, which are relevant for electrochemical devices (for example, low- and intermediate-temperature fuel cells) or which are of fundamental interest (such as molecular water acting as dopant in a lithium halide), are treated in more detail. Interrelations between hydration and electronic defects are also briefly touched upon.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Coulson, C. A. Valence (Oxford Univ. Press, 1961).
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999).
Agmon, N. et al. Protons and hydroxide ions in aqueous systems. Chem. Rev. 116, 7642–7672 (2016).
Munoz-Santiburcio, D. & Marx, D. Confinement-controlled aqueous chemistry within nanometric slit pores. Chem. Rev. 121, 6293–6320 (2021).
Maier, J. Nanoionics: ionic charge carriers in small systems. Phys. Chem. Chem. Phys. 11, 3011–3022 (2009).
Bunker, B. C. & Casey, W. H. The Aqueous Chemistry of Oxides (Oxford Univ. Press, 2016).
Maier, J. Acid–base centers and acid–base scales in ionic solids. Chem. Eur. J. 7, 4762–4770 (2001).
Marx, D. Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. ChemPhysChem 7, 1848–1870 (2006).
Hainovsky, N. & Maier, J. Simple phenomenological approach to premelting and sublattice melting in Frenkel disordered ionic crystals. Phys. Rev. B 51, 15789–15797 (1995).
Maier, J. & Munch, W. Thermal destiny of an ionic crystal. Z. Anorg. Allg. Chem. 626, 264–269 (2000).
Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. Science 283, 44–46 (1999).
Björneholm, O. et al. Water at interfaces. Chem. Rev. 116, 7698–7726 (2016).
Takada, K. et al. Superconductivity in two-dimensional CoO2 layers. Nature 422, 53–55 (2003).
Spaeth, M., Kreuer, K. D. & Maier, J. Giant Haven ratio for proton transport in sodium hydroxide. J. Solid State Chem. 148, 169–177 (1999).
Joos, M. et al. Lithium ion transport in water-containing Li(SCN) over a wide compositional range: from water doping to hydration. Solid State Ion. 394, 116130 (2023).
Armstrong, R. D. & Landles, K. Lithium ion conducting solids for ambient applications. J. Appl. Electrochem. 12, 533–535 (1982).
Nakamura, O. & Goodenough, J. B. Conductivity enhancement of lithium bromide monohydrate by Al2O3 particles. Solid State Ion. 7, 119–123 (1982).
Hartwig, P. & Weppner, W. Ionic conductivities of lithium-halide-based quaternary compounds. Solid State Ion. 3/4, 249–254 (1981).
Eigen, M. & DeMaeyer, L. Self-dissociation and protonic charge transport in water and ice. Proc. R. Soc. A 247, 505–533 (1958).
Kreuer, K. D., Rabenau, A. & Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. 21, 208–209 (1982).
Maier, J. Concentration polarization of salt-containing liquid electrolytes. Adv. Funct. Mater. 21, 1448–1455 (2011).
Li, X. et al. Water-mediated synthesis of a superionic halide solid electrolyte. Angew. Chem. Int. Ed. 58, 16427–16432 (2019).
Kreuer, K. D. Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003).
Sun, X. et al. Surface protonic conductivity in chemisorbed water in porous nanoscopic CeO2. Appl. Surf. Sci. 611, 155590 (2023).
Haile, S. M., Boysen, D. A., Chisholm, C. R. I. & Merle, R. B. Solid acids as fuel cell electrolytes. Nature 419, 910–913 (2001).
Wang, L. S., Patel, S. V., Truong, E., Hu, Y.-Y. & Haile, S. M. Phase behavior and superprotonic conductivity in the system (1 − x)CsH2PO4–xH3PO4: discovery of off-stoichiometric α-[Cs1−xHx]H2PO4. Chem. Mater. 34, 1809–1820 (2022).
Joos, M., Kang, X., Merkle, R. & Maier, J. Further reading and extended materials examples for the impact of water incorporation on ion transport in solids. Edmond https://doi.org/10.17617/3.2ELASF (2024).
Mitchell, J. B. et al. Confined interlayer water promotes structural stability for high-rate electrochemical proton intercalation in tungsten oxide hydrates. ACS Energy Lett. 4, 2805–2812 (2019).
Mitchell, J. A., Chagnot, M. & Augustyn, V. Hydrous transition metal oxides for electrochemical energy and environmental applications. Annu. Rev. Mater. Res. 53, 1–23 (2023).
Bubnova, O. et al. Semi-metallic polymers. Nat. Mater. 13, 190–194 (2014).
Paulsen, B. D., Fabiano, S. & Rivnay, J. Mixed ionic–electronic transport in polymers. Annu. Rev. Mater. Res. 51, 73–99 (2021).
Wieland, M., Dingler, C., Merkle, R., Maier, J. & Ludwigs, S. Humidity-controlled water uptake and conductivities in ion and electron mixed conducting polythiophene films. ACS Appl. Mater. Interfaces 12, 6742–6751 (2020).
Stotz, S. & Wagner, C. Die Löslichkeit von Wasserdampf und Wasserstoff in festen Oxiden. Ber. Bunsenges. Phys. Chem. 70, 781–788 (1966).
Iwahara, H., Esaka, T., Uchida, H. & Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 3/4, 359–363 (1981).
Ryu, K. H. & Haile, S. M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 125, 355–367 (1999).
Bjorheim, T. S., Hoedl, M. F., Merkle, R., Kotomin, E. A. & Maier, J. Proton, hydroxide ion, and oxide ion affinities of closed-shell metal oxides: importance for the hydration reaction and correlation to electronic structure. J. Phys. Chem. C 124, 1277–1284 (2020).
Kreuer, K. D., Schönherr, E. & Maier, J. Proton and oxygen diffusion in BaCeO3 based compounds: a combined thermal gravimetric analysis and conductivity study. Solid State Ion. 70, 278–284 (1994).
Münch, W., Seifert, G., Kreuer, K. D. & Maier, J. A quantum molecular dynamics study of proton conduction phenomena in BaCeO3. Solid State Ion. 86, 647–652 (1996).
Geneste, G. Proton transfer in barium zirconate: lattice reorganization, Landau–Zener curve-crossing approach. Solid State Ion. 232, 172–202 (2018).
Duan, C., Huang, J., Sullivan, N. & O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 7, 011314 (2020).
Strandbakke, R. et al. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 278, 120–132 (2015).
Papac, M., Stevanovic, V., Zakutayev, A. & O’Hayre, R. Triple ionic–electronic conducting oxides for next-generation electrochemical devices. Nat. Mater. 20, 301–313 (2021).
Merkle, R., Hoedl, M. F., Raimondi, G., Zohourian, R. & Maier, J. Oxides with mixed protonic and electronic conductivity. Annu. Rev. Mater. Res. 51, 461–493 (2021).
Raimondi, G. et al. Interplay of chemical, electronic and structural effects in the triple-conducting BaFeO3–Ba(Zr,Y)O3 solid solution. Chem. Mater. 35, 8945–8957 (2023).
Han, D., Okumura, Y., Nose, Y. & Uda, T. Synthesis of La1−xSrxSc1−yFeyO3−δ (LSSF) and measurement of water content in LSSF, LSCF and LSC hydrated in wet artificial air at 300 °C. Solid State Ion. 181, 1601–1606 (2010).
Yu, J. H., Lee, J. S. & Maier, J. Peculiar nonmonotonic water incorporation in oxides detected by local in situ optical absorption spectroscopy. Angew. Chem. Int. Ed. 46, 8992–8994 (2007).
Yoo, H.-I., Yoon, J.-Y., Ha, J.-S. & Lee, C.-E. Hydration and oxidation kinetics of a proton conductor oxide, SrCe0.95Yb0.05O2.975. Phys. Chem. Chem. Phys. 10, 974–982 (2008).
Merkle, R., Sitte, W. & Maier, J. Water incorporation into materials with three mobile carriers: two-fold relaxation of the electromotive force in Fe-doped SrTiO3 and importance of hole trapping. Solid State Ion. 347, 115174 (2020).
Kreuer, K. D. Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ion. 125, 285–302 (1999).
Kim, D. H., Kim, B. K. & Kim, Y. C. Energy barriers for proton migration in yttrium-doped barium zirconate super cell with Sigma 5 (310)/[001] tilt grain boundary. Solid State Ion. 213, 18–21 (2012).
Iguchi, F., Chen, C. T., Yugami, H. & Kim, S. Direct evidence of potential barriers at grain boundaries in Y-doped BaZrO3 from dc-bias dependence measurements. J. Mater. Chem. 21, 16517–16523 (2011).
Kjolseth, C. et al. Space-charge theory applied to the grain boundary impedance of proton conducting BaZr0.9Y0.1O3−d. Solid State Ion. 181, 268–275 (2010).
Shirpour, M., Merkle, R., Lin, C. T. & Maier, J. Nonlinear electrical grain boundary properties in proton conducting Y-BaZrO3 supporting the space charge depletion model. Phys. Chem. Chem. Phys. 14, 730–740 (2012).
Gregori, G., Merkle, R. & Maier, J. Ion conduction and redistribution at interfaces in oxide systems. Prog. Mater Sci. 89, 252–305 (2017).
Jia, C. L. & Urban, K. Atomic-resolution measurement of oxygen concentration in oxide materials. Science 303, 2001–2004 (2004).
Nyman, B. J., Helgee, E. E. & Wahnstrom, G. Oxygen vacancy segregation and space-charge effects in grain boundaries of dry and hydrated BaZrO3. Appl. Phys. Lett. 100, 061903 (2102).
Lindman, A., Bjorheim, T. S. & Wahnstrom, G. Defect segregation to grain boundaries in BaZrO3 from first-principles free energy calculations. J. Mater. Chem. A 5, 13421–13429 (2017).
Iguchi, F., Sata, N., Tsurui, T. & Yugami, H. Microstructures and grain boundary conductivity of BaZr1−xYxO3 (x = 0.05, 0.10, 0.15) ceramics. Solid State Ion. 178, 691–695 (2007).
Shirpour, M. et al. Dopant segregation and space charge effects in proton-conducting BaZrO3 perovskites. J. Phys. Chem. C 116, 2453–2461 (2012).
Clark, D. R. et al. Probing grain-boundary chemistry and electronic structure in proton-conducting oxides by atom probe tomography. Nano Lett. 16, 6924–6930 (2016).
Huang, Y., Merkle, R. & Maier, J. Effects of NiO addition on sintering and proton uptake of Ba(Zr,Ce,Y)O3−δ. J. Mater. Chem. A 9, 14775–14785 (2021).
Ngabonziza, P. et al. 2D doping of proton conductors: BaZrO3-based heterostructures. Adv. Energy Mater. 11, 2003267 (2021).
Draber, F. M. et al. Nanoscale percolation in doped BaZrO3 for high proton mobility. Nat. Mater. 19, 338–347 (2020).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Kreuer, K.-D. & Portale, G. A critical revision of the nano-morphology of proton conducting ionomers and polyelectrolytes for fuel cell applications. Adv. Funct. Mater. 23, 5390–5397 (2013).
Shin, D. W., Guiver, M. D. & Lee, Y. M. Hydrocarbon-based polymer electrolyte membranes: importance of morphology on ion transport and membrane stability. Chem. Rev. 117, 4759–4805 (2017).
Kreuer, K. D. Ion conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 26, 361–380 (2014).
Varcoe, J. R. et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 7, 3135–3191 (2014).
Kreuer, K. D. The role of internal pressure for the hydration and transport properties of ionomers and polyelectrolytes. Solid State Ion. 252, 93–101 (2013); corrigendum 328, 35–36 (2018).
Miyake, J. et al. Design of flexible polyphenylene proton-conducting membrane for next-generation fuel cells. Sci. Adv. 3, eaao0476 (2017).
Dekel, D. R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 375, 158–169 (2018).
Xue, J. et al. Toward alkaline‑stable anion exchange membranes in fuel cells: cycloaliphatic quaternary ammonium‑based anion conductors. Electrochem. Energy Rev. 5, 348–400 (2022).
Mardle, P., Chen, B. & Holdcroft, S. Opportunities of ionomer development for anion-exchange membrane water electrolysis. ACS Energy Lett. 8, 3330–3342 (2023).
Schuster, M., Kreuer, K.-D., Andersen, H. T. & Maier, J. Sulfonated poly(phenylene sulfone) polymers as hydrolytically and thermooxidatively stable proton conducting ionomers. Macromolecules 40, 598–607 (2007).
Bae, B., Miyatake, K. & Watanabe, M. Sulfonated poly(arylene ether sulfone ketone) multiblock copolymers with highly sulfonated block. synthesis and properties. Macromolecules 43, 2684–2691 (2010).
Kraytsberg, A. & Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 28, 7303–7330 (2014).
Kreuer, K.-D., Wohlfarth, A., de Araujo, C. C., Fuchs, A. & Maier, J. Single alkaline-ion (Li+, Na+) conductors by ion exchange of proton-conducting ionomers and polyelectrolytes. ChemPhysChem 12, 2258–2260 (2011).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013).
Bhattacharyya, A. J. & Maier, J. Second phase effects on the conductivity of non-aqueous salt solutions: ‘soggy sand electrolytes’. Adv. Mater. 16, 811–814 (2004).
Deng, R. et al. ‘Soggy-sand’ chemistry for high-voltage aqueous zinc-ion batteries. Adv. Mater. 36, 2311153 (2024).
Xue, Z., Heb, D. & Xie, X. Poly(ethylene oxide)-based electrolytes for lithium ion batteries. J. Mater. Chem. A 3, 19218–19253 (2015).
Whittingham, M. S. Hydrogen motion in oxides: from insulators to bronzes. Solid State Ion. 168, 255–263 (2004).
Joos, M. et al. Impact of hydration on ion transport in Li2Sn2S5xH2O. J. Mater. Chem. A 9, 16532–16544 (2021).
Hatz, A.-K. et al. Fast water-assisted lithium ion conduction in restacked lithium tin sulfide nanosheets. Chem. Mater. 33, 7337–7349 (2021).
Simon, U. & Franke, M. E. Electrical properties of nanoscaled host/guest compounds. Micropor. Mesopor. Mater. 41, 1–36 (2000).
Lim, D. W. & Kitagawa, H. Proton transport in metal–organic frameworks. Chem. Rev. 120, 8416–8467 (2020).
Jache, B. & Adelhelm, P. Use of graphite as a highly reversible electrode with superior cycle life for sodium-ion batteries by making use of co-intercalation phenomena. Angew. Chem. Int. Ed. 53, 10169–10173 (2014).
Park, J., Xu, Z.-L. & Kang, K. Solvated ion intercalation in graphite: sodium and beyond. Front. Chem. 8, 432 (2020).
Joos, M. et al. On the crystal structures of lithium thiocyanate monohydrate LiSCN⋅1H2O and the phase diagram LiSCN–H2O. J. Phys. Chem. Solids 160, 110299 (2022).
Suo, L. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Jeon, J. & Cho, M. Ion transport in super-concentrated aqueous electrolytes for lithium-ion batteries. J. Phys. Chem. C 125, 23622–23633 (2021).
Han, K. S. et al. Origin of unusual acidity and Li+ diffusivity in a series of water-in-salt electrolytes. J. Phys. Chem. B 124, 5284–5291 (2020).
Guo, Y. et al. Ion transport in semi-solid in-salt electrolytes: LiTFSI–H2O as a model system. J. Mater. Chem. A 11, 3427–3436 (2023).
Xu, J. et al. Aqueous electrolyte design for super-stable 2.5 V LiMn2O4 || Li4Ti5O12 pouch cells. Nat. Energy 7, 186–193 (2022).
Stub, S. Ø., Vøllestad, E. & Norby, T. Mechanisms of protonic surface transport in porous oxides: example of YSZ. J. Phys. Chem. C 121, 12817–12825 (2017).
Kim, S. et al. On the conduction pathway for protons in nanocrystalline yttria-stabilized zirconia. Phys. Chem. Chem. Phys. 11, 3035–3038 (2009).
Raz, S., Sasaki, K., Maier, J. & Riess, I. Characterization of adsorbed water layers on Y2O3-doped ZrO2. Solid State Ion. 143, 181–204 (2001).
Hinterberg, J. et al. 1H-NMR measurements of proton mobility in nano-crystalline YSZ. Phys. Chem. Chem. Phys. 15, 19825–19830 (2013).
Gregori, G., Shirpour, M. & Maier, J. Proton conduction in dense and porous nanocrystalline ceria thin films. Adv. Funct. Mater. 23, 5861–5867 (2013).
Sato, R., Ohkuma, S., Shibuta, Y., Shimojo, F. & Yamaguchi, S. Proton migration on hydrated surface of cubic ZrO2: ab initio molecular dynamics simulation. J. Phys. Chem. C 119, 28925–28933 (2015).
England, W. A., Gross, M. G., Hamnett, A., Wiseman, P. J. & Goodenough, J. B. Fast proton conduction in inorganic ion-exchange compounds. Solid State Ion. 1, 231–249 (1980).
Yang, J., Youssef, M. & Yildiz, B. Charged species redistribution at electrochemical interfaces: a model system of the zirconium oxide/water interface. Phys. Chem. Chem. Phys. 25, 6380–6391 (2023).
Norby, T., Sun, X. & Vøllestad, E. A brick layer model for surface conduction in porous ceramics. Solid State Ion. 398, 116269 (2023).
Fleig, J. & Maier, J. The impedance of imperfect electrode contacts on solid electrolytes. Solid State Ion. 85, 17–24 (1996).
Maier, J. Chemical potential of charge carriers in solids. Z. Phys. Chem. 219, 35–46 (2005).
Israelachvili, J. N. & Pashley, R. M. Molecular layering of water at surfaces and origin of repulsive hydration forces. Nature 306, 249–250 (1983).
Krämer, A., Vossen, M. & Forstmann, F. The influence of image interactions on the structure of water and electrolytes in front of a metal surface. J. Chem. Phys. 106, 2792–2800 (1997).
Fenter, P. & Sturchio, N. C. Mineral–water interfacial structures revealed by synchrotron X-ray scattering. Prog. Surf. Sci. 77, 171–258 (2004).
Gao, Z., Giovambattista, N. & Sahin, O. Phase diagram of water confined by graphene. Sci. Rep. 8, 6228 (2018).
Knight, A. W., Kalugin, N. G., Coker, E. & Ilgen, A. G. Water properties under nano-scale confinement. Sci. Rep. 9, 8246 (2019).
Gonella, G. et al. Water at charged interfaces. Nat. Rev. Chem. 5, 466–485 (2021).
Kreuer, K.-D., Paddison, S. J., Spohr, E. & Schuster, M. Transport in proton conductors for fuel-cell applications: simulations, elementary reactions, and phenomenology. Chem. Rev. 104, 4637–4678 (2004).
MacKinnon, R. Potassium channels and the atomic basis of selective ion conduction. Angew. Chem. Int. Ed. 43, 4265–4277 (2004).
Gelenter, M. D. et al. Water orientation and dynamics in the closed and open influenza B virus M2 proton channels. Commun. Biol. 4, 338 (2021).
Hara, S. & Miyayama, M. Proton conductivity of superacidic sulfated zirconia. Solid State Ion. 168, 111–116 (2004).
Aliouane, N. et al. Investigation of hydration and protonic conductivity of H-montmorillonite. Solid State Ion. 148, 103–110 (2002).
Felica, V. & Tavares, A. C. Faujasite zeolites as solid electrolyte for low temperature fuel cells. Solid State Ion. 194, 53–61 (2011).
Peng, Y. et al. Mechanoassisted synthesis of sulfonated covalent organic frameworks with high intrinsic proton conductivity. ACS Appl. Mater. Interfaces 8, 18505–18512 (2016).
Morris, D. R. & Sun, X. Water-sorption and transport properties of Nafion 117 H. J. Appl. Polym. Sci. 50, 1445–1452 (1993).
Liu, J., He, X., Zhang, J. Z. & Qi, L.-W. Hydrogen-bond structure dynamics in bulk water: insights from ab initio simulations with coupled cluster theory. Chem. Sci. 9, 2065–2073 (2018).
Babilo, P., Uda, T. & Haile, S. M. Processing of yttrium-doped barium zirconate for high proton conductivity. J. Mater. Res. 22, 1322–1330 (2007).
Maier, J. Physical Chemistry of Ionic Materials 2nd edn (Wiley, 2023)
Kröger, F. A. Chemistry of Imperfect Crystals (North–Holland, 1964)
Rickert, H. Electrochemistry of Solids (Springer, 1982).
Maier, J. Building versus structure elements: ionic charge carriers in solids. Z. Phys. Chem. 226, 863–870 (2012).
Xiao, C., Chen, C.-C. & Maier, J. Discrete modeling of space charge zones in solids. Phys. Chem. Chem. Phys. 24, 11945–11957 (2022).
Acknowledgements
We thank numerous colleagues in the Max Planck Institute for Solid State Research and the ‘Solid State Ionics’ community for valuable discussions concerning hydration effects and proton transport in a large range of materials. Graphics support by C. Blaga, and reading of the proofs by C. Schneider, are appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Truls Norby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joos, M., Kang, X., Merkle, R. et al. Water uptake of solids and its impact on ion transport. Nat. Mater. 24, 821–834 (2025). https://doi.org/10.1038/s41563-025-02143-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02143-8