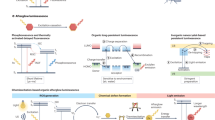
Abstract
Afterglow imaging is an emerging optical modality using agents that emit long-lasting luminescence after excitation ceases to eliminate tissue autofluorescence and improve signal-to-background ratios, achieving high imaging sensitivity and deep tissue penetration. Here we review recent advances in molecular afterglow imaging for biomedical applications, highlighting the materials and mechanisms involved in afterglow imaging modalities induced by light, ultrasound and ionizing radiation, termed photoafterglow, sonoafterglow and radioafterglow, respectively. We describe strategies to modulate the lifetime, intensity and wavelength of afterglow materials and principles for designing afterglow imaging probes that feature biomarker-activatable signal readouts and optimal biophysical properties for in vivo applications. We also highlight the applications of afterglow materials in disease diagnosis, imaging-guided therapy and in vitro diagnostics, and discuss the current challenges in the clinical translation of these technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
19 September 2025
In the version of the article initially published, there was an error in the first affiliation which has now been corrected to read "School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore, Singapore" in the HTML and PDF versions of the article.
References
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
James, M. L. & Gambhir, S. S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012).
Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photon. 8, 723–730 (2014).
Koch, M., Symvoulidis, P. & Ntziachristos, V. Tackling standardization in fluorescence molecular imaging. Nat. Photon. 12, 505–515 (2018).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Chen, Y., Wang, S. & Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 1, 60–78 (2023).
An, Z. et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 14, 685–690 (2015).
Zhen, X. et al. Ultralong phosphorescence of water-soluble organic nanoparticles for in vivo afterglow imaging. Adv. Mater. 29, 1606665 (2017).
He, Z. et al. Achieving persistent, efficient, and robust room‐temperature phosphorescence from pure organics for versatile applications. Adv. Mater. 31, 1807222 (2019).
Xiao, F. et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
Ran, C. & Pu, K. Molecularly generated light and its biomedical applications. Angew. Chem. Int. Ed. 63, e202314468 (2024).
le Masne de Chermont, Q. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl Acad. Sci. USA 104, 9266–9271 (2007).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Palner, M., Pu, K., Shao, S. & Rao, J. Semiconducting polymer nanoparticles with persistent near-infrared luminescence for in vivo optical imaging. Angew. Chem. Int. Ed. 54, 11477–11480 (2015).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Xu, C. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics. Nat. Biomed. Eng. 7, 298–312 (2023).
Huang, J. et al. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat. Mater. 22, 1421–1429 (2023).
Kobayashi, H., Ogawa, M., Alford, R., Choyke, P. L. & Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 110, 2620–2640 (2010).
Maldiney, T. et al. Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging. J. Am. Chem. Soc. 133, 11810–11815 (2011).
Abdukayum, A., Chen, J. T., Zhao, Q. & Yan, X. P. Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 135, 14125–14133 (2013).
Pan, Z., Lu, Y. Y. & Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr(3+)-doped zinc gallogermanates. Nat. Mater. 11, 58–63 (2011).
Li, Z. et al. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 137, 5304–5307 (2015).
Huang, K. et al. Three-dimensional colloidal controlled growth of core-shell heterostructured persistent luminescence nanocrystals. Nano Lett. 21, 4903–4910 (2021).
Yang, F. et al. A biomineral-inspired approach of synthesizing colloidal persistent phosphors as a multicolor, intravital light source. Sci. Adv. 8, eabo6743 (2022).
Wang, J., Li, J., Yu, J., Zhang, H. & Zhang, B. Large hollow cavity luminous nanoparticles with near-infrared persistent luminescence and tunable sizes for tumor afterglow imaging and chemo-/photodynamic therapies. ACS Nano 12, 4246–4258 (2018).
Wang, J. et al. Facile and controllable synthesis of the renal-clearable “luminous pearls” for in vivo afterglow/magnetic resonance imaging. ACS Nano 16, 462–472 (2021).
Li, Z. et al. In vivo repeatedly charging near-infrared-emitting mesoporous SiO2/ZnGa2O4:Cr3+ persistent luminescence nanocomposites. Adv. Sci. 2, 1500001 (2015).
Li, Z. et al. Enhancing rechargeable persistent luminescence via organic dye sensitization. Angew. Chem. Int. Ed. Engl 60, 15886–15890 (2021).
Xu, Y. et al. An aggregation-induced emission dye-powered afterglow luminogen for tumor imaging. Chem. Sci. 11, 419–428 (2020).
Jiang, Y. et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
Wei, X. et al. Highly bright near-infrared chemiluminescent probes for cancer imaging and laparotomy. Angew. Chem. Int. Ed. 62, e202213791 (2023).
Yang, J. et al. Turn-on chemiluminescence probes and dual-amplification of signal for detection of amyloid beta species in vivo. Nat. Commun. 11, 4052 (2020).
Li, Y. et al. Single‐component photochemical afterglow near‐infrared luminescent nano‐photosensitizers: bioimaging and photodynamic therapy. Adv. Health. Mater. 13, 2304392 (2024).
Li, J. et al. Building highly light‐harvesting near‐infrared aiegens using triazole‐based luminescent core for improved intravital afterglow imaging. Adv. Funct. Mater. 33, 2212380 (2023).
Cui, D., Xie, C., Li, J., Lyu, Y. & Pu, K. Semiconducting photosensitizer-incorporated copolymers as near-infrared afterglow nanoagents for tumor imaging. Adv. Health. Mater. 7, e1800329 (2018).
Liao, S. et al. A novel afterglow nanoreporter for monitoring cancer therapy. Theranostics 12, 6883 (2022).
Yang, L. et al. A highly bright near-infrared afterglow luminophore for activatable ultrasensitive in vivo imaging. Angew. Chem. Int. Ed. 63, e202313117 (2024).
Su, X. et al. Enhanced blue afterglow through molecular fusion for bio-applications. Angew. Chem. Int. Ed. 61, e202201630 (2022).
Zheng, G. S. et al. Photooxidation triggered ultralong afterglow in carbon nanodots. Nat. Commun. 15, 2365 (2024).
Chen, W. et al. Near-infrared afterglow luminescence of chlorin nanoparticles for ultrasensitive in vivo imaging. J. Am. Chem. Soc. 144, 6719–6726 (2022).
Zhu, J. et al. A self-sustaining near-infrared afterglow chemiluminophore for high-contrast activatable imaging. Angew. Chem. Int. Ed. 63, e202318545 (2024).
Duan, X. et al. Activatable persistent luminescence from porphyrin derivatives and supramolecular probes with imaging-modality transformable characteristics for improved biological applications. Angew. Chem. Int. Ed. 61, e202116174 (2022).
Juengpanich, S. et al. Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer. Nat. Commun. 14, 5699 (2023).
Liu, Y., Teng, L., Lou, X. F., Zhang, X. B. & Song, G. Four-in-one” design of a hemicyanine-based modular scaffold for high-contrast activatable molecular afterglow imaging. J. Am. Chem. Soc. 145, 5134–5144 (2023).
Li, Z. et al. Superoxide anion-mediated afterglow mechanism-based water-soluble zwitterion dye achieving renal-failure mice detection. J. Am. Chem. Soc. 145, 26736–26746 (2023).
Lawrence, J. P. Physics and instrumentation of ultrasound. Crit. Care Med. 35, S314–S322 (2007).
Baker, K. G., Robertson, V. J. & Duck, F. A. A review of therapeutic ultrasound: biophysical effects. Phys. Ther. 81, 1351–1358 (2001).
Cafarelli, A. et al. Piezoelectric nanomaterials activated by ultrasound: the pathway from discovery to future clinical adoption. ACS Nano 15, 11066–11086 (2021).
Van der Heggen, D. et al. Persistent luminescence in strontium aluminate: a roadmap to a brighter future. Adv. Funct. Mater. 32, 2208809 (2022).
Zhou, D. et al. Ultrasound-activated persistent luminescence imaging and bacteria-triggered drug release for Helicobacter pylori infection theranostics. ACS Appl. Mater. Interf. 14, 26418–26430 (2022).
Zhang, Z. et al. Ultrasound-chargeable persistent luminescence nanoparticles to generate self-propelled motion and photothermal/NO therapy for synergistic tumor treatment. ACS Nano 17, 16089–16106 (2023).
Wu, R. et al. Ultrasound-activated NIR chemiluminescence for deep tissue and tumor foci imaging. Anal. Chem. 95, 11219–11226 (2023).
Wang, W. et al. Ultrasound triggered organic mechanoluminescence materials. Adv. Drug Deliv. Rev. 186, 114343 (2022).
Wang, Y. et al. In vivo ultrasound-induced luminescence molecular imaging. Nat. Photon. 18, 334–343 (2024).
Son, S. et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 49, 3244–3261 (2020).
Alessio, A. M., Kinahan, P. E., Cheng, P. M., Vesselle, H. & Karp, J. S. PET/CT scanner instrumentation, challenges, and solutions. Radiol. Clin. North Am. 42, 1017–1032 (2004).
Chen, X., Song, J., Chen, X. & Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 48, 3073–3101 (2019).
Chen, H. et al. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horiz. 4, 1092–1101 (2017).
Chen, Z. Z. et al. Low dose of X-ray-excited long-lasting luminescent concave nanocubes in highly passive targeting deep-seated hepatic tumors. Adv. Mater. 31, e1905087 (2019).
Ding, D. et al. X-ray-activated simultaneous near-infrared and short-wave infrared persistent luminescence imaging for long-term tracking of drug delivery. ACS Appl. Mater. Interf. 13, 16166–16172 (2021).
Wang, X. et al. Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy. Nat. Commun. 13, 5091 (2022).
Huang, J. et al. Chemiluminescent probes with long-lasting high brightness for in vivo imaging of neutrophils. Angew. Chem. Int. Ed. 61, e202203235 (2022).
Tannous, R. et al. Spirostrain-accelerated chemiexcitation of dioxetanes yields unprecedented detection sensitivity in chemiluminescence bioassays. ACS Cent. Sci. 10, 28–42 (2024).
Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photon. 15, 187–192 (2021).
Li, L. et al. Mechanism of the trivalent lanthanides’ persistent luminescence in wide bandgap materials. Light. Sci. Appl. 11, 51 (2022).
Huang, J., Jiang, Y., Li, J., Huang, J. & Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. 60, 3999–4003 (2021).
Wang, X. & Pu, K. Molecular substrates for the construction of afterglow imaging probes in disease diagnosis and treatment. Chem. Soc. Rev. 52, 4549–4566 (2023).
Lei, L. et al. Noninvasive imaging of tumor glycolysis and chemotherapeutic resistance via de novo design of molecular afterglow scaffold. J. Am. Chem. Soc. 145, 24386–24400 (2023).
Jiang, Y. et al. Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 15, 2124 (2024).
Xu, C. et al. Activatable sonoafterglow nanoprobes for T-cell imaging. Adv. Mater. 35, e2211651 (2023).
Zeng, W. et al. An activatable afterglow/MRI bimodal nanoprobe with fast response to H2S for in vivo imaging of acute hepatitis. Angew. Chem. Int. Ed. 61, e202111759 (2022).
Huang, W. et al. Ratiometric afterglow luminescent imaging of matrix metalloproteinase-2 activity via an energy diversion process. Angew. Chem. Int. Ed. 63, e202404244 (2024).
Liu, Y. et al. Ratiometric afterglow luminescent nanoplatform enables reliable quantification and molecular imaging. Nat. Commun. 13, 2216 (2022).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
Xie, C., Zhen, X., Miao, Q., Lyu, Y. & Pu, K. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumors. Adv. Mater. 30, e1801331 (2018).
Maldiney, T. et al. Effect of core diameter, surface coating, and PEG chain length on the biodistribution of persistent luminescence nanoparticles in mice. ACS Nano 5, 854–862 (2011).
Kong, J., Zou, R., Law, G. L. & Wang, Y. Biomimetic multifunctional persistent luminescence nanoprobes for long-term near-infrared imaging and therapy of cerebral and cerebellar gliomas. Sci. Adv. 8, eabm7077 (2022).
Cabral, H., Li, J., Miyata, K. & Kataoka, K. Controlling the biodistribution and clearance of nanomedicines. Nat. Rev. Bioeng. 2, 214–232 (2024).
Xu, C. & Pu, K. Artificial urinary biomarker probes for diagnosis. Nat. Rev. Bioeng. 2, 425–441 (2024).
Luo, X. et al. Near-infrared persistent luminescence nanoprobe for early detection of atherosclerotic plaque. ACS Nano 18, 6500–6512 (2024).
Chen, C. et al. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Wei, X. et al. Leveraging long-distance singlet-oxygen transfer for bienzyme-locked afterglow imaging of intratumoral granule enzymes. J. Am. Chem. Soc. 146, 17393–17403 (2024).
He, S., Xie, C., Jiang, Y. & Pu, K. An organic afterglow protheranostic nanoassembly. Adv. Mater. 31, e1902672 (2019).
Gao, Z. et al. An activatable near-infrared afterglow theranostic prodrug with self-sustainable magnification effect of immunogenic cell death. Angew. Chem. Int. Ed. 61, e202209793 (2022).
Du, S., Yan, J., Xue, Y., Zhong, Y. & Dong, Y. Adoptive cell therapy for cancer treatment. Exploration 3, 20210058 (2023).
Ma, G. et al. Rechargeable afterglow nanotorches for in vivo tracing of cell-based microrobots. Angew. Chem. Int. Ed. 63, e202400658 (2024).
Gawne, P. J., Man, F., Blower, P. J. & de Rosales, R. T. M. Direct cell radiolabeling for in vivo cell tracking with PET and SPECT imaging. Chem. Rev. 122, 10266–10318 (2022).
Zhao, H. et al. Persistent luminescent nanoparticles containing hydrogels for targeted, sustained, and autofluorescence-free tumor metastasis imaging. Nano Lett. 20, 252–260 (2020).
Wu, S. Q., Yang, C. X. & Yan, X. P. A dual‐functional persistently luminescent nanocomposite enables engineering of mesenchymal stem cells for homing and gene therapy of glioblastoma. Adv. Funct. Mater. 27, 1604992 (2017).
Liu, J. et al. Tumor-microenvironment-activatable polymer nano-immunomodulator for precision cancer photoimmunotherapy. Adv. Mater. 34, e2106654 (2022).
Zhang, Y., Xu, C., Yang, X. & Pu, K. Photoactivatable protherapeutic nanomedicine for cancer. Adv. Mater. 32, e2002661 (2020).
He, S. et al. A semiconducting iron-chelating nano-immunomodulator for specific and sensitized sono-metallo-immunotherapy of cancer. Angew. Chem. Int. Ed. 62, e202310178 (2023).
Yu, J. et al. Polymeric STING pro-agonists for tumor-specific sonodynamic immunotherapy. Angew. Chem. Int. Ed. 62, e202307272 (2023).
Wang, Y. et al. Enhancing fractionated cancer therapy: a triple-anthracene photosensitizer unleashes long-persistent photodynamic and luminous efficacy. J. Am. Chem. Soc. 146, 6252–6265 (2024).
Wang, Y. et al. Cyclic amplification of the afterglow luminescent nanoreporter enables the prediction of anti-cancer efficiency. Angew. Chem. Int. Ed. 60, 19779–19789 (2021).
Wen, Y., Zhang, S., Yuan, W., Feng, W. & Li, F. Afterglow/fluorescence dual-emissive ratiometric oxygen probe for tumor hypoxia imaging. Anal. Chem. 95, 2478–2486 (2023).
Fan, W. et al. Enhanced afterglow performance of persistent luminescence implants for efficient repeatable photodynamic therapy. ACS Nano 11, 5864–5872 (2017).
Xu, C. & Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 50, 1111–1137 (2021).
Xu, M. et al. Activatable immunoprotease nanorestimulator for second near-infrared photothermal immunotherapy of cancer. ACS Nano 17, 8183–8194 (2023).
Qu, R. et al. Afterglow/photothermal bifunctional polymeric nanoparticles for precise postbreast-conserving surgery adjuvant therapy and early recurrence theranostic. Nano Lett. 23, 4216–4225 (2023).
Zhen, X., Xie, C. & Pu, K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angew. Chem. Int. Ed. 57, 3938–3942 (2018).
Chen, C., Zhang, X., Gao, Z., Feng, G. & Ding, D. Preparation of AIEgen-based near-infrared afterglow luminescence nanoprobes for tumor imaging and image-guided tumor resection. Nat. Protoc. 19, 2408–2434 (2024).
Ni, X. et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Gubala, V., Harris, L. F., Ricco, A. J., Tan, M. X. & Williams, D. E. Point of care diagnostics: status and future. Anal. Chem. 84, 487–515 (2012).
Lyu, Y. et al. Near-infrared afterglow semiconducting nano-polycomplexes for the multiplex differentiation of cancer exosomes. Angew. Chem. Int. Ed. 58, 4983–4987 (2019).
Timilsina, S. S., Jolly, P., Durr, N., Yafia, M. & Ingber, D. E. Enabling multiplexed electrochemical detection of biomarkers with high sensitivity in complex biological samples. Acc. Chem. Res. 54, 3529–3539 (2021).
Wu, B. Y., Wang, H. F., Chen, J. T. & Yan, X. P. Fluorescence resonance energy transfer inhibition assay for alpha-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles. J. Am. Chem. Soc. 133, 686–688 (2011).
Wang, J. et al. One-dimensional luminous nanorods featuring tunable persistent luminescence for autofluorescence-free biosensing. ACS Nano 11, 8185–8191 (2017).
Wang, X. et al. A persistent luminescence resonance energy transfer-based molecular beacon probe for the highly sensitive detection of microRNA in biological samples. Biosens. Bioelectron. 198, 113849 (2022).
Chen, X. et al. Self-assembled colloidal gold superparticles to enhance the sensitivity of lateral flow immunoassays with sandwich format. Theranostics 10, 3737–3748 (2020).
Lei, H., Wang, K., Ji, X. & Cui, D. Contactless measurement of magnetic nanoparticles on lateral flow strips using tunneling magnetoresistance (TMR) sensors in differential configuration. Sensors 16, 2130 (2016).
Wang, F., Zhong, Y., Bruns, O., Liang, Y. & Dai, H. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photon. 18, 535–547 (2024).
Gu, L. et al. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 4, 2326 (2013).
Fan, Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 13, 941–946 (2018).
Yang, Y. et al. NIR-II chemiluminescence molecular sensor for in vivo high-contrast inflammation imaging. Angew. Chem. Int. Ed. 59, 18380–18385 (2020).
Lu, L. et al. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
Li, Z. et al. Coloring afterglow nanoparticles for high-contrast time-gating-free multiplex luminescence imaging. Adv. Mater. 32, e2003881 (2020).
Lei, L. et al. Manipulation of time-dependent multicolour evolution of X-ray excited afterglow in lanthanide-doped fluoride nanoparticles. Nat. Commun. 13, 5739 (2022).
Angello, N. H. et al. Closed-loop transfer enables artificial intelligence to yield chemical knowledge. Nature 633, 351–358 (2024).
Wang, G., Ye, J. C. & De Man, B. Deep learning for tomographic image reconstruction. Nat. Mach. Intell. 2, 737–748 (2020).
Cheng, P. & Pu, K. Enzyme-responsive, multi-lock optical probes for molecular imaging and disease theranostics. Chem. Soc. Rev. 53, 10171–10188 (2024).
Naahidi, S. et al. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 166, 182–194 (2013).
Liew, S. S. et al. Renal-clearable molecular probe for near-infrared fluorescence imaging and urinalysis of SARS-CoV-2. J. Am. Chem. Soc. 143, 18827–18831 (2021).
He, S., Cheng, P. & Pu, K. Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine. Nat. Biomed. Eng. 7, 281–297 (2023).
Soenen, S. J. et al. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6, 446–465 (2011).
Maldiney, T. et al. Gadolinium‐doped persistent nanophosphors as versatile tool for multimodal in vivo imaging. Adv. Funct. Mater. 25, 331–338 (2015).
van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat. Med. 17, 1315–1319 (2011).
Hu, Z. et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 4, 259–271 (2020).
Acknowledgements
Y.Z. thanks the National Natural Science Foundation of China (22322406) for financial support. G.L. thanks the National Natural Science Foundation of China (22234002) for financial support. K.P. thanks the Singapore National Research Foundation (NRF-NRFI07-2021-0005) and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE-T2EP30220-0010 and MOE-T2EP30221-0004) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Xiaoyuan Chen, Hak Soo Choi, Fan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Zhang, Y., Liang, G. et al. Molecular afterglow imaging for biomedical applications. Nat. Mater. (2025). https://doi.org/10.1038/s41563-025-02338-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-025-02338-z