Abstract
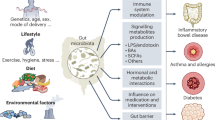
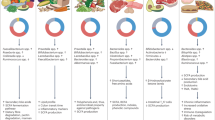
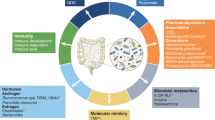
Dietary patterns and specific dietary components, in concert with the gut microbiota, can jointly shape susceptibility, resistance and therapeutic response to cancer. Which diet–microbial interactions contribute to or mitigate carcinogenesis and how they work are important questions in this growing field. Here we interpret studies of diet–microbial interactions to assess dietary determinants of intestinal colonization by opportunistic and oncogenic bacteria. We explore how diet-induced expansion of specific gut bacteria might drive colonic epithelial tumorigenesis or create immuno-permissive tumour milieus and introduce recent findings that provide insight into these processes. Additionally, we describe available preclinical models that are widely used to study diet, microbiome and cancer interactions. Given the rising clinical interest in dietary modulations in cancer treatment, we highlight promising clinical trials that describe the effects of different dietary alterations on the microbiome and cancer outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cordain, L. et al. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 81, 341–354 (2005).
Yang, J. et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology 162, 135–149.e2 (2022).
Schulz, M. D. et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512 (2014).
Newsome, R., Yang, Y. & Jobin, C. Western diet influences on microbiome and carcinogenesis. Semin. Immunol. 67, 101756 (2023).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Byndloss, M. X. et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Lee, J.-Y. et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe 28, 273–284.e6 (2020).
Cevallos, S. A. et al. Increased epithelial oxygenation links colitis to an expansion of tumorigenic bacteria. mBio 10, e02244-19 (2019).
Cao, Y. et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 378, eabm3233 (2022).
He, Z. et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 68, 289–300 (2019).
Van Elsland, D. M. et al. Repetitive non-typhoidal Salmonella exposure is an environmental risk factor for colon cancer and tumor growth. Cell Rep. Med. 3, 100852 (2022).
El Tekle, G. & Garrett, W. S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 23, 600–618 (2023).
Lee, J.-Y., Tsolis, R. M. & Bäumler, A. J. The microbiome and gut homeostasis. Science 377, eabp9960 (2022).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
O’Keefe, S. J. D. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6, 6342 (2015).
Mehta, R. S. et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3, 921–927 (2017).
Liu, L. et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain Fusobacterium nucleatum. Clin. Gastroenterol. Hepatol. 16, 1622–1631.e3 (2018).
Arima, K. et al. Western-style diet, pks island-carrying Escherichia coli, and colorectal cancer: analyses from two large prospective cohort studies. Gastroenterology 163, 862–874 (2022).
Wilson, M. R. et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785 (2019).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020).
Buc, E. et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 8, e56964 (2013).
Guidi, R. et al. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response: bacterial toxin and genomic instability. Cell Microbiol. 15, 98–113 (2013).
Warren, R. L. et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 1, 16 (2013).
Nakatsu, G. et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 6, 8727 (2015).
Liu, Y. et al. Bacterial genotoxin accelerates transient infection-driven murine colon tumorigenesis. Cancer Discov. 12, 236–249 (2022).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 6, e145207 (2021).
Nagpal, R., Neth, B. J., Wang, S., Craft, S. & Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542 (2019).
Zhu, W. et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med. 216, 2378–2393 (2019).
Zhu, W. et al. Precision editing of the gut microbiota ameliorates colitis. Nature 553, 208–211 (2018).
Holscher, H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184 (2017).
Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393, 434–445 (2019).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Kunzmann, A. T. et al. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 102, 881–890 (2015).
Gonzalez, D. & Mavridou, D. A. I. Making the best of aggression: the many dimensions of bacterial toxin regulation. Trends Microbiol. 27, 897–905 (2019).
Sears, C. L., Geis, A. L. & Housseau, F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J. Clin. Invest. 124, 4166–4172 (2014).
Casterline, B. W., Hecht, A. L., Choi, V. M. & Bubeck Wardenburg, J. The Bacteroides fragilis pathogenicity island links virulence and strain competition. Gut Microbes 8, 374–383 (2017).
Brennan, C. A. et al. Aspirin modulation of the colorectal cancer-associated microbe Fusobacterium nucleatum. mBio 12, e00547-21 (2021).
Brennan, C. A. & Garrett, W. S. Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166 (2019).
Martin, P. et al. Interplay between siderophores and colibactin genotoxin biosynthetic pathways in Escherichia coli. PLoS Pathog. 9, e1003437 (2013).
Oliero, M. et al. Oligosaccharides increase the genotoxic effect of colibactin produced by pks+ Escherichia coli strains. BMC Cancer 21, 172 (2021).
Oliero, M. et al. Inulin impacts tumorigenesis promotion by colibactin-producing Escherichia coli in ApcMin/+ mice. Front. Microbiol. 14, 1067505 (2023).
Moen, B. et al. Effect of dietary fibers on cecal microbiota and intestinal tumorigenesis in azoxymethane treated A/J Min/+ mice. PLoS ONE 11, e0155402 (2016).
Muñoz-Esparza, N. C. et al. Polyamines in food. Front. Nutr. 6, 108 (2019).
Chagneau, C. V. et al. The polyamine spermidine modulates the production of the bacterial genotoxin colibactin. mSphere 4, e00414-19 (2019).
Boling, L. et al. Dietary prophage inducers and antimicrobials: toward landscaping the human gut microbiome. Gut Microbes 11, 721–734 (2020).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Riboli, E. et al. Carcinogenicity of aspartame, methyleugenol, and isoeugenol. Lancet Oncol. 24, 848–850 (2023).
Oh, J.-H. et al. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe 25, 273–284.e6 (2019).
Silpe, J. E., Wong, J. W. H., Owen, S. V., Baym, M. & Balskus, E. P. The bacterial toxin colibactin triggers prophage induction. Nature 603, 315–320 (2022).
Balasubramanian, S., Osburne, M. S., BrinJones, H., Tai, A. K. & Leong, J. M. Prophage induction, but not production of phage particles, is required for lethal disease in a microbiome-replete murine model of enterohemorrhagic E. coli infection. PLoS Pathog. 15, e1007494 (2019).
Dumitrescu, D. G. et al. A microbial transporter of the dietary antioxidant ergothioneine. Cell 185, 4526–4540.e18 (2022).
Borodina, I. et al. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 33, 190–217 (2020).
Ba, D. M. et al. Higher mushroom consumption is associated with lower risk of cancer: a systematic review and meta-analysis of observational studies. Adv. Nutr. 12, 1691–1704 (2021).
D’Onofrio, N. et al. Diet‐derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 596, 1313–1329 (2022).
Wang, H. et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 34, 581–594.e8 (2022).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022).
Li, D. et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 116, 230–243 (2022).
Haikonen, R., Kärkkäinen, O., Koistinen, V. & Hanhineva, K. Diet- and microbiota-related metabolite, 5-aminovaleric acid betaine (5-AVAB), in health and disease. Trends Endocrinol. Metab. 33, 463–480 (2022).
Goedert, J. J. et al. Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis 35, 2089–2096 (2014).
Zeller, G. et al. Potential of fecal microbiota for early‐stage detection of colorectal cancer. Mol. Syst. Biol. 10, 766 (2014).
Sakanaka, A. et al. Fusobacterium nucleatum metabolically integrates commensals and pathogens in oral biofilms. mSystems 7, e00170-22 (2022).
Aymeric, L. et al. Colorectal cancer specific conditions promote Streptococcus gallolyticus gut colonization. Proc. Natl Acad. Sci. USA 115, E283–E291 (2018).
Queen, J. et al. Comparative analysis of colon cancer-derived Fusobacterium nucleatum subspecies: inflammation and colon tumorigenesis in murine models. mBio 13, e02991-21 (2022).
Dejea, C. M. et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl Acad. Sci. USA 111, 18321–18326 (2014).
Missiaglia, E. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 25, 1995–2001 (2014).
Goodwin, A. C. et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl Acad. Sci. USA 108, 15354–15359 (2011).
Ding, N. et al. Fusobacterium nucleatum infection induces malignant proliferation of esophageal squamous cell carcinoma cell by putrescine production. Microbiol. Spectr. 11, e02759-22 (2023).
Farriol, M., Segovia-Silvestre, T., Castellanos, J. M., Venereo, Y. & Orta, X. Role of putrescine in cell proliferation in a colon carcinoma cell line. Nutrition 17, 934–938 (2001).
Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Shipitsin, M. & Polyak, K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab. Invest. 88, 459–463 (2008).
Kaiko, G. E. et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016).
Xing, P. Y., Pettersson, S. & Kundu, P. Microbial metabolites and intestinal stem cells tune intestinal homeostasis. Proteomics 20, 1800419 (2020).
Wu, S. et al. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 586, 108–112 (2020).
Donohoe, D. R. et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 4, 1387–1397 (2014).
Belcheva, A. et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158, 288–299 (2014).
Yang, W. et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11, 4457 (2020).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Chun, E. et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 51, 871–884.e6 (2019).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Dupraz, L. et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 36, 109332 (2021).
Lee, Y.-S. et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24, 833–846.e6 (2018).
Hou, Y. et al. A diet–microbial metabolism feedforward loop modulates intestinal stem cell renewal in the stressed gut. Nat. Commun. 12, 271 (2021).
Taylor, S. R. et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature 597, 263–267 (2021).
Kadosh, E. et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586, 133–138 (2020).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28, 2344–2352 (2022).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023).
Park, E. M. et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703 (2022).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
Ji, M. et al. Methionine restriction-induced sulfur deficiency impairs antitumour immunity partially through gut microbiota. Nat. Metab. 5, 1526–1543 (2023).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Matson, V. & Gajewski, T. F. Dietary modulation of the gut microbiome as an immunoregulatory intervention. Cancer Cell 40, 246–248 (2022).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Lee, K. A. et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 28, 535–544 (2022).
Nomura, M. et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 3, e202895 (2020).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019).
Coutzac, C. et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020).
Goncalves, M. D. et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019).
Ferrer, M. et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 35, 1147–1162.e7 (2023).
Dmitrieva-Posocco, O. et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 605, 160–165 (2022).
Satoh, T. New prebiotics by ketone donation. Trends Endocrinol. Metab. 34, 414–425 (2023).
Sasaki, K., Sasaki, D., Hannya, A., Tsubota, J. & Kondo, A. In vitro human colonic microbiota utilises d-β-hydroxybutyrate to increase butyrogenesis. Sci. Rep. 10, 8516 (2020).
Ferrer, M. et al. Cachexia: a systemic consequence of progressive, unresolved disease. Cell 186, 1824–1845 (2023).
Han, J.-X. et al. Microbiota-derived tryptophan catabolites mediate the chemopreventive effects of statins on colorectal cancer. Nat. Microbiol. 8, 919–933 (2023).
Zhang, Y.-G. et al. Vitamin D receptor protects against dysbiosis and tumorigenesis via the JAK/STAT pathway in intestine. Cell. Mol. Gastroenterol. Hepatol. 10, 729–746 (2020).
Sun, L. et al. Bile salt hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal cancer. Nat. Commun. 14, 755 (2023).
Pang, J. et al. Resveratrol intervention attenuates chylomicron secretion via repressing intestinal FXR-induced expression of scavenger receptor SR-B1. Nat. Commun. 14, 2656 (2023).
Mishra, A., Giuliani, G. & Longo, V. D. Nutrition and dietary restrictions in cancer prevention. Biochim. Biophys. Acta Rev. Cancer 1879, 189063 (2024).
Collins, N. & Belkaid, Y. Control of immunity via nutritional interventions. Immunity 55, 210–223 (2022).
Rangan, P. et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 26, 2704–2719.e6 (2019).
Gordon, J. I., Dewey, K. G., Mills, D. A. & Medzhitov, R. M. The human gut microbiota and undernutrition. Sci. Transl. Med. 4, 137ps12 (2012).
Chang, H.-W. et al. Prevotella copri and microbiota members mediate the beneficial effects of a therapeutic food for malnutrition. Nat. Microbiol. 9, 922–937 (2024).
Tan-Shalaby, J. L. et al. Modified Atkins diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr. Metab. 13, 52 (2016).
Longo, V. D. & Fontana, L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol. Sci. 31, 89–98 (2010).
Hibberd, A. A. et al. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 4, e000145 (2017).
Frugé et al. Primary outcomes of a randomized controlled crossover trial to explore the effects of a high chlorophyll dietary intervention to reduce colon cancer risk in adults: the Meat and Three Greens (M3G) Feasibility Trial. Nutrients 11, 2349 (2019).
Aronson, W. J. et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J. Urol. 183, 345–350 (2010).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Kucherlapati, M. H. Mouse models in colon cancer, inferences, and implications. iScience 26, 106958 (2023).
Roper, J. et al. Colonoscopy-based colorectal cancer modeling in mice with CRISPR–Cas9 genome editing and organoid transplantation. Nat. Protoc. 13, 217–234 (2018).
Arnesen, H. et al. Induction of colorectal carcinogenesis in the C57BL/6J and A/J mouse strains with a reduced DSS dose in the AOM/DSS model. Lab. Anim. Res. 37, 19 (2021).
Saam, J. R. & Gordon, J. I. Inducible gene knockouts in the small intestinal and colonic epithelium. J. Biol. Chem. 274, 38071–38082 (1999).
El Marjou, F. et al. Tissue‐specific and inducible Cre‐mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Acknowledgements
This work is supported by a National Institutes of Health grant (R01CA154426) and the Cancer Research UK Grand Challenges Initiative (C10674/A27140) to W.S.G. We thank all W.S.G. laboratory members for helpful discussions and contributions.
Author information
Authors and Affiliations
Contributions
G.N., N.A. and W.S.G. wrote and edited the paper. M.H.M. generated the figures.
Corresponding author
Ethics declarations
Competing interests
W.S.G. is on the scientific advisory board of Empress Therapeutics, Freya Biosciences, Seres Therapeutics, Scipher Medicine and Sail Biosciences, all unrelated to this review article. W.S.G.’s laboratory receives funding from Merck and Astellas. The other authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Melek Arkan, K. Leigh Greathouse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakatsu, G., Andreeva, N., MacDonald, M.H. et al. Interactions between diet and gut microbiota in cancer. Nat Microbiol 9, 1644–1654 (2024). https://doi.org/10.1038/s41564-024-01736-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01736-4
This article is cited by
-
Genetic evidence for causal links between diet, gut microbiota, and hepatobiliary cancer: a Mendelian randomization study
AMB Express (2025)
-
Genetically proxied gut microbiota and cancer risk: a scoping review of Mendelian randomization studies
Archives of Public Health (2025)
-
Decoding microbe–diet–host synergy in colorectal cancer
Nature Microbiology (2025)
-
Microbiota-mediated mechanisms of mucosal immunity across the lifespan
Nature Immunology (2025)
-
Decoding the gut microbiota metabolite–matrix metalloproteinase-3 axis in breast cancer: a multi-omics and network pharmacology study
Molecular Diversity (2025)