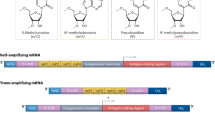
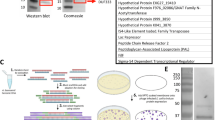
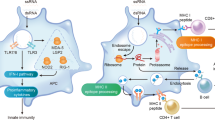
Abstract
The global surge in antimicrobial resistance presents a critical threat to public health, emphasizing the urgent need for the development of new and more effective bacterial vaccines. Since the success of mRNA vaccines during the COVID-19 pandemic, this vaccine strategy has rapidly advanced, with most efforts focused on cancer immunotherapy and targeting viral pathogens. Recently, mRNA vaccines have entered the early phases of clinical development for bacterial diseases. However, bacteria present greater biological complexity compared with viruses, posing additional challenges for vaccine design, such as antigen selection, immune response and mRNA construct design. Here, we discuss critical aspects in the development of bacterial mRNA vaccines, from antigen selection to construct design. We also highlight the current preclinical landscape and discuss remaining translational challenges and future potential for mRNA vaccines against bacterial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Patel, J. et al. Measuring the global response to antimicrobial resistance, 2020–21: a systematic governance analysis of 114 countries. Lancet Infect. Dis. 23, 706–718 (2023).
Urgent call for better use of existing vaccines and development of new vaccines to tackle AMR. World Health Organization https://www.who.int/news/item/12-07-2022-urgent-call-for-better-use-of-existing-vaccines-and-development-of-new-vaccines-to-tackle-amr (2022).
CDC. Antibiotic Resistance Threats in the United States 2019 (US Department of Health and Human Services, 2019).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Bacterial Vaccines in Clinical and Preclinical Development 2021 (World Health Organization, 2021).
Bloom, D. E., Black, S., Salisbury, D. & Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl Acad. Sci. USA 115, 12868–12871 (2018).
Frost, I. et al. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe 4, e113–e125 (2023).
Moore, M. R. et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir. Med. 4, 399–406 (2016).
Micoli, F., Bagnoli, F., Rappuoli, R. & Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302 (2021).
Baker, S. J., Payne, D. J., Rappuoli, R. & De Gregorio, E. Technologies to address antimicrobial resistance. Proc. Natl Acad. Sci. USA 115, 12887–12895 (2018).
Adepoju, P. New TB vaccine on trial. Nat. Africa 10.1038/d44148-024-00101-1 (2024).
Olivier, V. D. M. et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 379, 1621–1634 (2018).
Chen, W. Will the mRNA vaccine platform be the panacea for the development of vaccines against antimicrobial resistant (AMR) pathogens? Expert Rev. Vaccines 21, 155–157 (2022).
Mayer, R. L. & Impens, F. Immunopeptidomics for next-generation bacterial vaccine development. Trends Microbiol. 29, 1034–1045 (2021).
van der Woude, M. W. & Bäumler, A. J. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17, 581–611 (2004).
Tan, A., Atack, J. M., Jennings, M. P. & Seib, K. L. The capricious nature of bacterial pathogens: phasevarions and vaccine development. Front. Immunol. 7, 586 (2016).
Seib, K. L., Srikhanta, Y. N., Atack, J. M. & Jennings, M. P. Epigenetic regulation of virulence and immunoevasion by phase-variable restriction-modification systems in bacterial pathogens. Annu. Rev. Microbiol. 74, 655–671 (2020).
Trzilova, D., Anjuwon-Foster, B. R., Torres Rivera, D. & Tamayo, R. Rho factor mediates flagellum and toxin phase variation and impacts virulence in Clostridioides difficile. PLoS Pathog. 16, e1008708 (2020).
Georgieva, M., Kagedan, L., Ying-Jie, L., Thompson, C. M. & Lipsitch, M. Antigenic variation in Streptococcus pneumoniae PspC promotes immune escape in the presence of variant-specific immunity. mBio 9, e00264-18 (2018).
Vink, C., Rudenko, G. & Seifert, H. S. Microbial antigenic variation mediated by homologous DNA recombination. FEMS Microbiol. Rev. 36, 917–948 (2012).
Ernst, J. D. in Strain Variation in the Mycobacterium Tuberculosis Complex: Its Role in Biology, Epidemiology and Control (ed. Gagneux, S.) 171–190 (Springer, 2017).
Rappuoli, R. Glycoconjugate vaccines: principles and mechanisms. Sci. Transl. Med. 10, eaat4615 (2018).
Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 3, 445–450 (2000).
Sette, A. & Rappuoli, R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity 33, 530–541 (2010).
Mayer, R. L. et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 13, 6075 (2022).
Pardi, N. & Krammer, F. mRNA vaccines for infectious diseases—advances, challenges and opportunities. Nat. Rev. Drug Discov. 23, 838–861 (2024).
Nagata, T., Uchijima, M., Yoshida, A., Kawashima, M. & Koide, Y. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 261, 445–451 (1999).
Maruggi, G. et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 35, 361–368 (2017).
Kawaguchi, K. et al. mRNA vaccine induces protective immunity against the type III secretory virulence of Pseudomonas aeruginosa. Preprint at https://www.biorxiv.org/content/10.1101/2023.06.09.544431v1.full.pdf (2023).
Messou, M.-A. A. Harnessing a mRNA Platform Against Salmonellae (Harvard Univ. Press, 2018).
Kon, E. et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci. Adv. 9, eadg1036 (2023).
Ozdilek, A. & Avci, F. Y. Glycosylation as a key parameter in the design of nucleic acid vaccines. Curr. Opin. Struct. Biol. 73, 102348 (2022).
Gupta, R. & Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. In Proc. Pacific Symposium on Biocomputing 2002 (eds Altman, R. B. et al.) 310–322 (World Scientific, 2002).
Ozdilek, A., Paschall, A. V., Dookwah, M., Tiemeyer, M. & Avci, F. Y. Host protein glycosylation in nucleic acid vaccines as a potential hurdle in vaccine design for nonviral pathogens. Proc. Natl Acad. Sci. USA 117, 1280–1282 (2020).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013).
Ndiaye, B. P. et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 3, 190–200 (2015).
Tameris, M. et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine 33, 2944–2954 (2015).
Muir, E. et al. Trafficking and processing of bacterial proteins by mammalian cells: insights from chondroitinase ABC. PLoS ONE 12, e0186759 (2017).
Rabouille, C. Pathways of unconventional protein secretion. Trends Cell Biol. 27, 230–240 (2017).
Lin, P. L. & Flynn, J. A. L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 37, 239–249 (2015).
Grant, N. L. et al. Mycobacterium tuberculosis-specific CD4 T cells expressing transcription factors T-Bet or RORγT associate with bacterial control in granulomas. mBio 14, e00477-23 (2023).
Tian, L. et al. CTLs: killers of intracellular bacteria. Front. Cell. Infect. Microbiol. 12, 967679 (2022).
Osterloh, A. Vaccination against bacterial infections: challenges, progress and new approaches with a focus on intracellular bacteria. Vaccines 10, 751 (2022).
Shanmuganathan, G. et al. Role of interferons in Mycobacterium tuberculosis infection. Clin. Pract. 12, 788–796 (2022).
Baker, H. A. & Bernardini, J. P. It’s not just a phase; ubiquitination in cytosolic protein quality control. Biochem. Soc. Trans. 49, 365–377 (2021).
Weigele, B. A., Orchard, R. C., Jimenez, A., Cox, G. W. & Alto, N. M. A systematic exploration of the interactions between bacterial effector proteins and host cell membranes. Nat. Commun. 8, 532 (2017).
Dersh, D., Hollý, J. & Yewdell, J. W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 21, 116–128 (2021).
Ruiz Cuevas, M. V. et al. Most non-canonical proteins uniquely populate the proteome or immunopeptidome. Cell Rep. 34, 108815 (2021).
Yewdell, J. W. Hide and seek in the peptidome. Science 301, 1334–1335 (2003).
Pamer, E. G. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4, 812–823 (2004).
Delogu, G., Howard, A., Collins, F. M. & Morris, S. L. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect. Immun. 68, 3097–3102 (2000).
Pishesha, N., Harmand, T. J. & Ploegh, H. L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 22, 751–764 (2022).
Lizée, G., Basha, G. & Jefferies, W. A. Tails of wonder: endocytic-sorting motifs key for exogenous antigen presentation. Trends Immunol. 26, 141–149 (2005).
Lotteau, V. et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature 348, 600–605 (1990).
Rowell, J. F. et al. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J. Immunol. 155, 1818–1828 (1995).
Wu, T. C. et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc. Natl Acad. Sci. USA 92, 11671–11675 (1995).
Kreiter, S. et al. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 180, 309–318 (2008).
Lizée, G. et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat. Immunol. 4, 1065–1073 (2003).
Diken, M., Kranz, L. M., Kreiter, S. & Sahin, U. mRNA: a versatile molecule for cancer vaccines. Curr. Issues Mol. Biol. 22, 113–128 (2017).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
You, Z. et al. Induction of vigorous helper and cytotoxic T cell as well as B cell responses by dendritic cells expressing a modified antigen targeting receptor-mediated internalization pathway1. J. Immunol. 165, 4581–4591 (2000).
Noy-Porat, T. et al. Acetylcholinesterase-Fc fusion protein (AChE-Fc): a novel potential organophosphate bioscavenger with extended plasma half-life. Bioconjug. Chem. 26, 1753–1758 (2015).
Elia, U. et al. Design of SARS-CoV-2 hFc-conjugated receptor-binding domain mRNA vaccine delivered via lipid nanoparticles. ACS Nano 15, 9627–9637 (2021).
Shattock, R. J. et al. A self-amplifying RNA vaccine provides protection in a murine model of bubonic plague. Front. Microbiol. 14, 1247041 (2023).
Meulewaeter, S. et al. Alpha-galactosylceramide improves the potency of mRNA LNP vaccines against cancer and intracellular bacteria. J. Controlled Release 370, 379–391 (2024).
Wang, X. et al. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa. NPJ Vaccines 8, 76 (2023).
Mead, P. Epidemiology of Lyme disease. Infect. Dis. Clin. North Am. 36, 495–521 (2022).
Moderna announces clinical and program updates at 4th vaccines day. Moderna https://www.accesswire.com/748485/Moderna-Announces-Clinical-and-Program-Updates-at-4th-Vaccines-Day (2023).
Pine, M. et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 31, 2702–2714 (2023).
Gross, D. M. et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281, 703–706 (1998).
Bobe, J. R. et al. Recent progress in Lyme disease and remaining challenges. Front. Med. 8, 666554 (2021).
Wolf, M. A. et al. Multivalent mRNA-DTP vaccines are immunogenic and provide protection from Bordetella pertussis challenge in mice. NPJ Vaccines 9, 103 (2024).
Global Tuberculosis Report 2023 (World Health Organization, 2023).
Mangtani, P. et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 58, 470–480 (2014).
Larsen, S. E. et al. An RNA-based vaccine platform for use against Mycobacterium tuberculosis. Vaccines 11, 130 (2023).
Neha, A., Albrecht, S. S., Mustafa, D., Annette, V. & Ugur, S (2024). RNA for preventing or treating tuberculosis. WIPO patent WO2024028445A1 (2023).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Verbeke, R., Hogan, M. J., Loré, K. & Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 55, 1993–2005 (2022).
Morais, P., Adachi, H. & Yu, Y.-T. The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Front. Cell. Dev. Biol. 9, 789427 (2021).
Mulroney, T. E. et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 625, 189–194 (2024).
Rappuoli, R., Alter, G. & Pulendran, B. Transforming vaccinology. Cell 187, 5171–5194 (2024).
Petit, T. J. P. & Lebreton, A. Adaptations of intracellular bacteria to vacuolar or cytosolic niches. Trends Microbiol. 30, 736–748 (2022).
Leddy, O., White, F. M. & Bryson, B. D. Immunopeptidomics reveals determinants of Mycobacterium tuberculosis antigen presentation on MHC class I. eLife 12, e84070 (2023).
Panda, S. et al. Identification of differentially recognized T cell epitopes in the spectrum of tuberculosis infection. Nat. Commun. 15, 765 (2024).
Sette, A. & Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 310, 27–46 (2022).
Arunachalam, P. S. et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Invest. 133, e167955 (2023).
Fedele, G., Palmieri, A. & Onder, G. The immune response to SARS-CoV-2 vaccination in older people. Lancet Healthy Longev. 4, e177–e178 (2023).
Kamar, N. et al. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 385, 661–662 (2021).
Walsh, E. E. et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 388, 1465–1477 (2023).
Pennington, S. H. et al. Nonspecific effects of oral vaccination with live-attenuated Salmonella Typhi strain Ty21a. Sci. Adv. 5, eaau6849 (2024).
Kleinnijenhuis, J. et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Ziogas, A., Bruno, M., van der Meel, R., Mulder, W. J. M. & Netea, M. G. Trained immunity: target for prophylaxis and therapy. Cell Host Microbe 31, 1776–1791 (2023).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Yamaguchi, Y. et al. Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells. JCI Insight 7, e163347 (2022).
Benn, C. S., Schaltz-Buchholzer, F., Nielsen, S., Netea, M. G. & Aaby, P. Randomized clinical trials of COVID-19 vaccines: do adenovirus-vector vaccines have beneficial non-specific effects? iScience 26, 106733 (2023).
Föhse, K. et al. The impact of BNT162b2 mRNA vaccine on adaptive and innate immune responses. Clin. Immunol. 255, 109762 (2023).
Stevens, N. E. et al. No evidence of durable trained immunity after two doses of adenovirus-vectored or mRNA COVID-19 vaccines. J. Clin. Invest. 133, e171742 (2023).
Hellgren, F. et al. Modulation of innate immune response to mRNA vaccination after SARS-CoV-2 infection or sequential vaccination in humans. JCI Insight 9, e175401 (2024).
Han, X. et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 18, 1105–1114 (2023).
Hayashizaki, K., Kamii, Y. & Kinjo, Y. Glycolipid antigen recognition by invariant natural killer T cells and its role in homeostasis and antimicrobial responses. Front. Immunol. 15, 1402412 (2024).
Pifferi, C., Fuentes, R. & Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 5, 197–216 (2021).
Gozzi, N. et al. Estimating the impact of COVID-19 vaccine inequities: a modeling study. Nat. Commun. 14, 3272 (2023).
Leppek, K. et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 13, 1536 (2022).
Meulewaeter, S. et al. Continuous freeze-drying of messenger RNA lipid nanoparticles enables storage at higher temperatures. J. Control. Release 357, 149–160 (2023).
Muramatsu, H. et al. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 30, 1941–1951 (2022).
Saied, A. A. Building Africa’s first mRNA vaccine facility. Lancet 402, 287–288 (2023).
Xue, T. et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect. Immun. 72, 6324–6329 (2004).
Lorenzi, J. C. C. et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis. BMC Biotechnol. 10, 77 (2010).
Blakney, A. K. et al. Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J. Control. Release 304, 65–74 (2019).
Arya, S., Lin, Q., Zhou, N., Gao, X. & Huang, J. D. Strong immune responses induced by direct local injections of modified mRNA-lipid nanocomplexes. Mol. Ther. Nucleic Acids 19, 1098–1109 (2020).
Sajid, A. et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 13, 9827 (2021).
Rais, M. et al. Immunogenicity and protection against Mycobacterium avium with a heterologous RNA prime and protein boost vaccine regimen. Tuberculosis 138, 102302 (2023).
Legere, R. M. et al. Intramuscular but not nebulized administration of a mRNA vaccine against Rhodococcus equi stimulated humoral immune responses in neonatal foals. Am. J. Vet. Res. 85, ajvr.23.09.0208 (2023).
Alameh, M.-G. et al. A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection. Science 386, 69–75 (2024).
Leddy, O., White, F. & Bryson, B. Leveraging immunopeptidomics to study and combat infectious disease. mSystems 6, 10–21 (2021).
Leddy, O. et al. Immunopeptidomics informs discovery and delivery of Mycobacterium tuberculosis MHC-II antigens for vaccine design. Preprint at https://www.biorxiv.org/content/10.1101/2024.10.02.616386v1.full (2024).
Leddy, O., Yuki, Y., Carrington, M., Bryson, B. D. & White, F. M. PathMHC: a workflow to selectively target pathogen-derived MHC peptides in discovery immunopeptidomics experiments for vaccine target identification. Preprint at https://www.biorxiv.org/content/10.1101/2024.09.11.612454v1.full.pdf (2024).
Gul, A. et al. Immunopeptidomics mapping of Listeria monocytogenes T cell epitopes in mice. Mol. Cell. Proteom. 23, 100829 (2024).
Rodríguez-Ortega, M. J. et al. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat. Biotechnol. 24, 191–197 (2006).
Montemari, A. L. et al. A shaving proteomic approach to unveil surface proteins modulation of multi-drug resistant Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Front. Med. 9, 818669 (2022).
Menikou, S. et al. A proteomics-based method for identifying antigens within immune complexes. PLoS ONE 15, e0244157 (2021).
Joglekar, A. V. & Li, G. T cell antigen discovery. Nat. Methods 18, 873–880 (2021).
Musvosvi, M. et al. T cell receptor repertoires associated with control and disease progression following Mycobacterium tuberculosis infection. Nat. Med. 29, 258–269 (2023).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38, 1194–1202 (2020).
Mora, M., Donati, C., Medini, D., Covacci, A. & Rappuoli, R. Microbial genomes and vaccine design: refinements to the classical reverse vaccinology approach. Curr. Opin. Microbiol. 9, 532–536 (2006).
He, Y., Xiang, Z. & Mobley, H. L. T. Vaxign: The first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J. Biomed. Biotechnol. 2010, 297505 (2010).
Vivona, S., Bernante, F. & Filippini, F. NERVE: New Enhanced Reverse Vaccinology Environment. BMC Biotechnol. 6, 35 (2006).
Rawal, K. et al. Vaxi-DL: a web-based deep learning server to identify potential vaccine candidates. Comput. Biol. Med. 145, 105401 (2022).
Ong, E. et al. Vaxign-ML: supervised machine learning reverse vaccinology model for improved prediction of bacterial protective antigens. Bioinformatics 36, 3185–3191 (2020).
Peters, B., Nielsen, M. & Sette, A. T cell epitope predictions. Annu. Rev. Immunol. 38, 123–145 (2020).
Sanchez-Trincado, J. L., Gomez-Perosanz, M. & Reche, P. A. Fundamentals and methods for T- and B-cell epitope prediction. J. Immunol. Res. 2017, 2680160 (2017).
De Groot, A. S. et al. Better epitope discovery, precision immune engineering, and accelerated vaccine design using Immunoinformatics tools. Front. Immunol. 11, 442 (2020).
Lindestam Arlehamn, C. S. et al. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3 + CCR6 + Th1 subset. PLoS Pathog. 9, e1003130 (2013).
da Silva Antunes, R. et al. T cell reactivity to Bordetella pertussis is highly diverse regardless of childhood vaccination. Cell Host Microbe 31, 1404–1416.e4 (2023).
Mubarak, A. S., Ameen, Z. S., Hassan, A. S. & Ozsahin, D. U. Enhancing tuberculosis vaccine development: a deconvolution neural network approach for multi-epitope prediction. Sci. Rep. 14, 10375 (2024).
Shi, J. et al. In silico designed novel multi-epitope mRNA vaccines against Brucella by targeting extracellular protein BtuB and LptD. Sci. Rep. 14, 7278 (2024).
Prachar, M. et al. Identification and validation of 174 COVID-19 vaccine candidate epitopes reveals low performance of common epitope prediction tools. Sci. Rep. 10, 20465 (2020).
Buckley, P. R. et al. Evaluating performance of existing computational models in predicting CD8+ T cell pathogenic epitopes and cancer neoantigens. Brief. Bioinform. 23, bbac141 (2022).
Conev, A., Fasoulis, R., Hall-Swan, S., Ferreira, R. & Kavraki, L. E. HLAEquity: examining biases in pan-allele peptide-HLA binding predictors. iScience 27, 108613 (2024).
Gonzalez-Galarza, F. F., Christmas, S., Middleton, D. & Jones, A. R. Allele Frequency Net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 39, D913–D919 (2011).
Zimmermann, P. The immunological interplay between vaccination and the intestinal microbiota. NPJ Vaccines 8, 24 (2023).
Acknowledgements
This work was supported by a Research Foundation-Flanders (FWO-Vlaanderen) PhD fellowship for strategic basic research to I.A. (1S40923N) and postdoctoral fellowships to R.V. (1275023N), F.T. (12AN524N) and P.W. (12T1722N). D.P. acknowledges support from the European Research Council (ERC adv. grant no. 101055029), The EXPERT project (European Union’s Horizon 2020 Research and Innovation Programme under grant no. 825828), ISF grant 2012/20 and the Shmunis Family Foundation. F.I. and I.L. acknowledge support from Ghent University Concerted Research Action grant BOF21/GOA/033 and from European Union’s Horizon Europe research and from the Horizon Europe Project BAXERNA 2.0 (101080544). F.I. is further supported by starting grant BOF/STA/202209/011 from Ghent University.
Author information
Authors and Affiliations
Contributions
I.A., R.V., P.W., F.T., F.I. and I.L.: conceptualization, visualization, drafting and review of the manuscript. U.E., S.C.D.S., R.R. and D.P.: review and editing.
Corresponding authors
Ethics declarations
Competing interests
D.P. receives licensing fees (to patents on which he was an inventor), has invested in, consults for (or is on scientific advisory boards or boards of directors) or is a founder and hold shares or conducts sponsored research at Tel Aviv University for the following entities: ART Biosciences, BioNTech SE, Earli Inc., Geneditor Biologics Inc. Kernal Biologics, Merck, Newphase Ltd, NeoVac Ltd, RiboX Therapeutics, Roche, SirTLabs Corporation and Teva Pharmaceuticals Inc. R.R. holds shares in the GSK and Novartis group of companies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Timothy Keys and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aernout, I., Verbeke, R., Thery, F. et al. Challenges and opportunities in mRNA vaccine development against bacteria. Nat Microbiol 10, 1816–1828 (2025). https://doi.org/10.1038/s41564-025-02070-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02070-z