Abstract
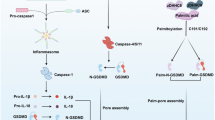
Intracellular bacterial pathogens can cause high levels of morbidity and mortality in humans. Host immune responses that protect against these infections include pyroptosis, a form of lytic cell death caused by the insertion of large gasdermin (GSDM) pores into the host plasma membrane. Here we review recent advances in our understanding of how the five GSDM proteins, GSDMA–E, are activated by distinct signalling pathways. Pyroptosis can both eliminate intracellular niches and release cytosolic interleukin-1 family cytokines that further prime host immune responses against the invading pathogen. Because pyroptosis targets microbes, host-adapted intracellular pathogens have evolved strategies to efficiently subvert it. However, environmental pathogens fail to evade, making pyroptosis a potent barrier against infection. We summarize recent findings for the host-adapted bacterial pathogens Shigella flexneri, Salmonella enterica serovar Typhimurium and Mycobacterium tuberculosis, contrasted with the environmental bacteria Burkholderia thailandensis and Chromobacterium violaceum.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Park, W. et al. Diversity and complexity of cell death: a historical review. Exp. Mol. Med. 55, 1573–1594 (2023).
Zychlinsky, A., Prevost, M. C. & Sansonetti, P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169 (1992).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
Remick, B. C., Gaidt, M. M. & Vance, R. E. Effector-triggered immunity. Annu. Rev. Immunol. 41, 453–481 (2023).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Kovacs, S. B. & Miao, E. A. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 27, 673–684 (2017).
Jorgensen, I., Zhang, Y., Krantz, B. A. & Miao, E. A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 213, 2113–2128 (2016).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Billman, Z. P. et al. Caspase-1 activates gasdermin A in non-mammals. eLife 12, RP92362 (2024).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Balasubramanian, A. et al. The palmitoylation of gasdermin D directs its membrane translocation and pore formation during pyroptosis. Sci. Immunol. 9, eadn1452 (2024).
Margheritis, E. et al. Gasdermin D cysteine residues synergistically control its palmitoylation-mediated membrane targeting and assembly. EMBO J. 43, 4274–4297 (2024).
Du, G. et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 630, 437–446 (2024).
Liu, Z. et al. Palmitoylation at a conserved cysteine residue facilitates gasdermin D-mediated pyroptosis and cytokine release. Proc. Natl Acad. Sci. USA 121, e2400883121 (2024).
Zhang, N. et al. A palmitoylation–depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis. Nat. Cell Biol. 26, 757–769 (2024).
Jiang, X. et al. NU6300 covalently reacts with cysteine-191 of gasdermin D to block its cleavage and palmitoylation. Sci. Adv. 10, eadi9284 (2024).
Hu, L. et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 11, 281 (2020).
Nozaki, K. & Miao, E. A. Bucket lists must be completed during cell death. Trends Cell Biol. 33, 803–815 (2023).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Defourny, J. et al. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl Acad. Sci. USA 116, 8010–8017 (2019).
Monteleone, M. et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 24, 1425–1433 (2018).
Nozaki, K., Li, L. & Miao, E. A. Innate sensors trigger regulated cell death to combat intracellular infection. Annu. Rev. Immunol. 40, 469–498 (2022).
Cayrol, C. & Girard, J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl Acad. Sci. USA 106, 9021–9026 (2009).
Lacey, C. A. & Miao, E. A. Programmed cell death in the evolutionary race against bacterial virulence factors. Cold Spring Harb. Perspect. Biol. 12, a036459 (2020).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Lee, B. L. et al. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 215, 2279–2288 (2018).
Wang, K. et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 180, 941–955 e920 (2020).
Gu, Y. et al. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997).
Exconde, P. M. et al. The tetrapeptide sequence of IL-18 and IL-1β regulates their recruitment and activation by inflammatory caspases. Cell Rep. 42, 113581 (2023).
Shi, X. et al. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature 624, 442–450 (2023).
Devant, P. et al. Structural insights into cytokine cleavage by inflammatory caspase-4. Nature 624, 451–459 (2023).
Annunziato, F., Romagnani, C. & Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 135, 626–635 (2015).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Taabazuing, C. Y., Okondo, M. C. & Bachovchin, D. A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–514. e504 (2017).
Demarco, B. et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 6, eabc3465 (2020).
Mihaly, S. R., Ninomiya-Tsuji, J. & Morioka, S. TAK1 control of cell death. Cell Death Differ. 21, 1667–1676 (2014).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018).
Nozaki, K. et al. Caspase-7 activates ASM to repair gasdermin and perforin pores. Nature 606, 960–967 (2022).
Ruhl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Wei, B., Billman, Z. P., Nozaki, K., Goodridge, H. S. & Miao, E. A. NLRP3, NLRP6 and NLRP12 are inflammasomes with distinct expression patterns. Front. Immunol. 15, 1418290 (2024).
Chen, K. W. et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 (2014).
Ulland, T. K. et al. Nlrp12 mutation causes C57BL/6J strain-specific defect in neutrophil recruitment. Nat. Commun. 7, 13180 (2016).
Cai, S., Batra, S., Del Piero, F. & Jeyaseelan, S. NLRP12 modulates host defense through IL-17A–CXCL1 axis. Mucosal Immunol. 9, 503–514 (2016).
Oh, C. et al. Neutrophil inflammasomes sense the subcellular delivery route of translocated bacterial effectors and toxins. Cell Rep. 41, 111688 (2022).
Oh, C., Spears, T. J. & Aachoui, Y. Inflammasome-mediated pyroptosis in defense against pathogenic bacteria. Immunol. Rev. 329, e13408 (2025).
Chen, K. W. et al. RIPK1 activates distinct gasdermins in macrophages and neutrophils upon pathogen blockade of innate immune signaling. Proc. Natl Acad.Sci. USA 118, e2101189118 (2021).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Rogers, C. et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017).
Zamaraeva, M. V. et al. Cells die with increased cytosolic ATP during apoptosis: a bioluminescence study with intracellular luciferase. Cell Death Differ. 12, 1390–1397 (2005).
Masuda, Y. et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J. Hum. Genet. 51, 652–664 (2006).
Chen, W. et al. Synthetic lethality of combined ULK1 defection and p53 restoration induce pyroptosis by directly upregulating GSDME transcription and cleavage activation through ROS/NLRP3 signaling. J. Exp. Clin. Cancer Res. 43, 248 (2024).
Zhu, Y. P. et al. NET formation is a default epigenetic program controlled by PAD4 in apoptotic neutrophils. Sci. Adv. 9, eadj1397 (2023).
Souza, F. W. & Miao, E. A. Neutrophils only die twice. Sci. Adv. 9, eadm8715 (2023).
Ma, F. et al. Gasdermin E dictates inflammatory responses by controlling the mode of neutrophil death. Nat. Commun. 15, 386 (2024).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Zhou, B. et al. Full-length GSDME mediates pyroptosis independent from cleavage. Nat. Cell Biol. 26, 1545–1557 (2024).
Wu, C. et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 10, R130 (2009).
Das, S. et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc. Natl Acad. Sci. USA 113, 13132–13137 (2016).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020).
Oltra, S. S. et al. Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. Cell Death Differ. 30, 1366–1381 (2023).
Li, X. et al. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 30, 2120–2134 (2023).
Chao, K. L., Kulakova, L. & Herzberg, O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl Acad. Sci. USA 114, E1128–E1137 (2017).
Zhong, X. et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature 616, 598–605 (2023).
Wang, C. et al. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature 616, 590–597 (2023).
Hansen, J. M. et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191.e3118 (2021).
Xi, R. et al. Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity. Proc. Natl Acad. Sci. USA 118, e2026307118 (2021).
Yang, L. et al. Intraepithelial mast cells drive gasdermin C-mediated type 2 immunity. Immunity 57, 1056–1070 e1055 (2024).
Zhao, M. et al. Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and gasdermin C. Immunity 55, 623–638.e625 (2022).
Choi, H. W. et al. Loss of bladder epithelium induced by cytolytic mast cell granules. Immunity 45, 1258–1269 (2016).
Hou, J. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 (2020).
Zhang, J. Y. et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 31, 980–997 (2021).
Jena, K. K. et al. Type III interferons induce pyroptosis in gut epithelial cells and impair mucosal repair. Cell 187, 7533–7550 e7523 (2024).
Luthi, A. U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
LaRock, D. L. et al. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 605, 527–531 (2022).
Deng, W. et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 (2022).
LaRock, C. N. et al. IL-1β is an innate immune sensor of microbial proteolysis. Sci. Immunol. 1, eaah3539 (2016).
Nelson, D. C., Garbe, J. & Collin, M. Cysteine proteinase SpeB from Streptococcus pyogenes—a potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 392, 1077–1088 (2011).
Persson, H., Vindebro, R. & von Pawel-Rammingen, U. The streptococcal cysteine protease SpeB is not a natural immunoglobulin-cleaving enzyme. Infect. Immun. 81, 2236–2241 (2013).
Yin, H. et al. Seneca Valley virus circumvents gasdermin A-mediated inflammation by targeting the pore-formation domain for cleavage. mBio 15, e0168024 (2024).
Vander Broek, C. W. & Stevens, J. M. Type III secretion in the melioidosis pathogen Burkholderia pseudomallei. Front. Cell. Infect. Microbiol. 7, 255 (2017).
Szczesna, M. et al. Bacterial esterases reverse lipopolysaccharide ubiquitylation to block host immunity. Cell Host Microbe 32, 913–924 e917 (2024).
Hernandez, D., Walsh, S., Saavedra Sanchez, L., Dickinson, M. S. & Coers, J. Interferon-inducible E3 ligase RNF213 facilitates host-protective linear and K63-linked ubiquitylation of Toxoplasma gondii parasitophorous vacuoles. mBio 13, e0188822 (2022).
Benanti, E. L., Nguyen, C. M. & Welch, M. D. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell 161, 348–360 (2015).
Plum, M. T. W. et al. Burkholderia thailandensis uses a type VI secretion system to lyse protrusions without triggering host cell responses. Cell Host Microbe 32, 676–692 e675 (2024).
Kostow, N. & Welch, M. D. Plasma membrane protrusions mediate host cell-cell fusion induced by Burkholderia thailandensis. Mol. Biol. Cell 33, ar70 (2022).
Dilucca, M., Ramos, S., Shkarina, K., Santos, J. C. & Broz, P. Guanylate-binding protein-dependent noncanonical inflammasome activation prevents Burkholderia thailandensis-induced multinucleated giant cell formation. mBio 12, e0205421 (2021).
Place, D. E. et al. Interferon inducible GBPs restrict Burkholderia thailandensis motility induced cell-cell fusion. PLoS Pathog. 16, e1008364 (2020).
Aachoui, Y. et al. Canonical inflammasomes drive IFN-γ to prime caspase-11 in defense against a cytosol-invasive bacterium. Cell Host Microbe 18, 320–332 (2015).
Kovacs, S. B. et al. Neutrophil caspase-11 is essential to defend against a cytosol-invasive bacterium. Cell Rep. 32, 107967 (2020).
Wang, J. et al. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog. 14, e1007105 (2018).
Wang, J., Deobald, K. & Re, F. Gasdermin D protects from melioidosis through pyroptosis and direct killing of bacteria. J. Immunol. 202, 3468–3473 (2019).
DuPont, H. L., Levine, M. M., Hornick, R. B. & Formal, S. B. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159, 1126–1128 (1989).
Allaoui, A., Mounier, J., Prévost, M. C., Sansonetti, P. J. & Parsot, C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol. Microbiol. 6, 1605–1616 (1992).
Campbell-Valois, F.-X., Sachse, M., Sansonetti, P. J. & Parsot, C. Escape of actively secreting Shigella flexneri from ATG8/LC3-positive vacuoles formed during cell-to-cell spread is facilitated by IcsB and VirA. mBio 6, e02567–14 (2015).
Liu, W. et al. N(ε)-fatty acylation of multiple membrane-associated proteins by Shigella IcsB effector to modulate host function. Nat. Microbiol. 3, 996–1009 (2018).
Saavedra-Sanchez, L. et al. The Shigella flexneri effector IpaH1.4 facilitates RNF213 degradation and protects cytosolic bacteria against interferon-induced ubiquitylation. eLife 13, RP102714 (2025).
Naydenova, K. et al. Shigella flexneri evades LPS ubiquitylation through IpaH1.4-mediated degradation of RNF213. Nat. Struct. Mol. Biol. 32, 1741–1751 (2025).
Li, P. et al. Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 551, 378–383 (2017).
Piro, A. S. et al. Detection of cytosolic Shigella flexnerivia a C-terminal triple-arginine motif of GBP1 inhibits actin-based motility. mBio 8, 01979–17 (2017).
Wandel, M. P. et al. GBPs inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin ligase IpaH9.8. Cell Host Microbe 22, 507–518.e505 (2017).
Dickinson, M. aryS. et al. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc. Natl Acad. Sci. USA 120, e2216028120 (2023).
Fisch, D. et al. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J. 38, e100926 (2019).
Kutsch, M. et al. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 (2020).
Luchetti, G. et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–1530.e1510 (2021).
Li, Z. et al. Shigella evades pyroptosis by arginine ADP-riboxanation of caspase-11. Nature 599, 290–295 (2021).
Liu, Y. et al. Calmodulin binding activates Chromobacterium CopC effector to ADP-riboxanate host apoptotic caspases. mBio 13, e006922 (2022).
Hou, Y. et al. Structural mechanisms of calmodulin activation of Shigella effector OspC3 to ADP-riboxanate caspase-4/11 and block pyroptosis. Nat. Struct. Mol. Biol. 30, 261–272 (2023).
Kobayashi, T. et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe 13, 570–583 (2013).
Ashida, H., Sasakawa, C. & Suzuki, T. A unique bacterial tactic to circumvent the cell death crosstalk induced by blockade of caspase-8. EMBO J. 39, e104469 (2020).
Tominaga, A., Mahmoud, M. A., Mukaihara, T. & Enomoto, M. Molecular characterization of intact, but cryptic, flagellin genes in the genus Shigella. Mol. Microbiol. 12, 277–285 (1994).
Turcotte, E. A. et al. Shigella OspF blocks rapid p38-dependent priming of the NAIP-NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 123, e2510950123 (2026).
Mitchell, P. S. et al. NAIP-NLRC4-deficient mice are susceptible to shigellosis. eLife 9, e59022 (2020).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Miao, E. A. & Rajan, J. V. Salmonella and caspase-1: a complex interplay of detection and evasion. Front. Microbiol. 2, 85 (2011).
Naseer, N. et al. Salmonella enterica serovar Typhimurium induces NAIP/NLRC4- and NLRP3/ASC-independent, caspase-4-dependent inflammasome activation in human intestinal epithelial cells. Infect. Immun. 90, e0066321 (2022).
Romberg, N., Vogel, T. P. & Canna, S. W. NLRC4 inflammasomopathies. Curr. Opin. Allergy Clin. Immunol. 17, 398–404 (2017).
Knodler, L. A. et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl Acad. Sci. USA 107, 17733–17738 (2010).
Sellin, M. E. et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16, 237–248 (2014).
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Rauch, I. et al. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659 (2017).
Crowley, S. M. et al. Intestinal restriction of Salmonella Typhimurium requires caspase-1 and caspase-11 epithelial intrinsic inflammasomes. PLoS Pathog. 16, e1008498 (2020).
Knodler, L. A., Nair, V. & Steele-Mortimer, O. Quantitative assessment of cytosolic Salmonella in epithelial cells. PLoS ONE 9, e84681 (2014).
Knodler, L. A. Salmonella enterica: living a double life in epithelial cells. Curr. Opin. Microbiol. 23, 23–31 (2015).
Powers, T. R. et al. Intracellular niche-specific profiling reveals transcriptional adaptations required for the cytosolic lifestyle of Salmonella enterica. PLoS Pathog. 17, e1009280 (2021).
Geiser, P. et al. Salmonella enterica serovar Typhimurium exploits cycling through epithelial cells to colonize human and murine enteroids. mBio 12, e02684–20 (2021).
Taylor, S. J. & Winter, S. E. Salmonella finds a way: metabolic versatility of Salmonella enterica serovar Typhimurium in diverse host environments. PLoS Pathog. 16, e1008540 (2020).
Batista, J. H. & da Silva Neto, J. F. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front. Microbiol. 8, 2213 (2017).
Macher, A. M., Casale, T. B. & Fauci, A. S. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann. Intern. Med. 97, 51–55 (1982).
Maltez, V. I. et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43, 987–997 (2015).
Miki, T. et al. Chromobacterium pathogenicity island 1 type III secretion system is a major virulence determinant for Chromobacterium violaceum-induced cell death in hepatocytes. Mol. Microbiol. 77, 855–872 (2010).
Peng, T. et al. Pathogen hijacks programmed cell death signaling by arginine ADPR-deacylization of caspases. Mol. Cell 82, 1806–1820 e1808 (2022).
Harvest, C. K. et al. An innate granuloma eradicates an environmental pathogen using Gsdmd and Nos2. Nat. Commun. 14, 6686 (2023).
Amason, M. E., Beatty, C. J., Harvest, C. K., Saban, D. R. & Miao, E. A. Chemokine expression profile of an innate granuloma. eLife 13, e96425 (2024).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
McElvania Tekippe, E. et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS ONE 5, e12320 (2010).
Chai, Q. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022).
Qu, Z. et al. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1β immune pathway. Sci. Adv. 6, eaba4733 (2020).
Beckwith, K. S. et al. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat. Commun. 11, 2270 (2020).
Koo, I. C. et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10, 1866–1878 (2008).
Kurenuma, T. et al. The RD1 locus in the Mycobacterium tuberculosis genome contributes to activation of caspase-1 via induction of potassium ion efflux in infected macrophages. Infect. Immun. 77, 3992–4001 (2009).
Yao, Q. et al. Lnc-EST12, which is negatively regulated by mycobacterial EST12, suppresses antimycobacterial innate immunity through its interaction with FUBP3. Cell. Mol. Immunol. 19, 883–897 (2022).
Koul, A. et al. Serine/threonine protein kinases PknF and PknG of Mycobacterium tuberculosis: characterization and localization. Microbiology 147, 2307–2314 (2001).
Deol, P. et al. Role of Mycobacterium tuberculosis Ser/Thr kinase PknF: implications in glucose transport and cell division. J. Bacteriol. 187, 3415–3420 (2005).
Rastogi, S., Ellinwood, S., Augenstreich, J., Mayer-Barber, K. D. & Briken, V. Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLoS Pathog. 17, e1009712 (2021).
Master, S. S. et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3, 224–232 (2008).
Mishra, B. B. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 14, 52–60 (2013).
Saiga, H. et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int. Immunol. 24, 637–644 (2012).
Shah, S. et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent Mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J. Immunol. 191, 3514–3518 (2013).
Araki, T. & Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 17, 353–361 (1996).
David, L. et al. NINJ1 mediates plasma membrane rupture by cutting and releasing membrane disks. Cell 187, 2224–2235 e2216 (2024).
Weinberg, J. M., Davis, J. A., Abarzua, M. & Rajan, T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J. Clin. Invest. 80, 1446–1454 (1987).
Borges, J. P. et al. J1 membrane clustering to suppress plasma membrane rupture in cell death. eLife 11, e78609 (2022).
Zhu, Y. et al. NINJ1 regulates plasma membrane fragility under mechanical strain. Nature 644, 1088–1096 (2025).
Wein, T. et al. CARD domains mediate anti-phage defence in bacterial gasdermin systems. Nature 639, 727–734 (2025).
Gao, L. A. et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science 377, eabm4096 (2022).
Xu, H., Yuan, Z., Qin, K., Jiang, S. & Sun, L. The molecular mechanism and evolutionary divergence of caspase 3/7-regulated gasdermin E activation. eLife 12, e89974 (2024).
Liu, J. et al. Chicken gasdermins mediate pyroptosis after the cleavage by caspases. Int. J. Biol. Macromol. 270, 132476 (2024).
Zhao, Y. et al. Activation mechanism of CcGSDMEb-1/2 and regulation for bacterial clearance in common carp (Cyprinus carpio). J. Immunol. 211, 658–672 (2023).
Thurston, T. L. et al. Growth inhibition of cytosolic Salmonella by caspase-1 and caspase-11 precedes host cell death. Nat. Commun. 7, 13292 (2016).
Huan, Y., Kong, Q., Mou, H. & Yi, H. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front. Microbiol. 11, 582779 (2020).
Dominguez-Medina, C. C. et al. Outer membrane protein size and LPS O-antigen define protective antibody targeting to the Salmonella surface. Nat. Commun. 11, 851 (2020).
Parsons, E. S. et al. Single-molecule kinetics of pore assembly by the membrane attack complex. Nat. Commun. 10, 2066 (2019).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Mulvihill, E. et al. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 37, e98321 (2018).
Kappelhoff, S. et al. Structure and regulation of GSDMD pores at the plasma membrane of pyroptotic cells. Preprint at bioRxiv https://doi.org/10.1101/2023.10.24.563742 (2023).
Benn, G. et al. Phase separation in the outer membrane of Escherichia coli. Proc. Natl Acad. Sci. USA 118, e2112237118 (2021).
Lithgow, T., Stubenrauch, C. J. & Stumpf, M. P. H. Surveying membrane landscapes: a new look at the bacterial cell surface. Nat. Rev. Microbiol. 21, 502–518 (2023).
Weindel, C. G. et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 185, 3214–3231 e3223 (2022).
Casanova, J. L., MacMicking, J. D. & Nathan, C. F. Interferon-γ and infectious diseases: lessons and prospects. Science 384, eadl2016 (2024).
Parker, M. E. et al. c-Maf regulates the plasticity of group 3 innate lymphoid cells by restraining the type 1 program. J. Exp. Med. 217, e20191030 (2020).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Victor, A. R. et al. IL-18 drives ILC3 proliferation and promotes IL-22 production via NF-kB. J. Immunol. 199, 2333–2342 (2017).
Stone, K. D., Prussin, C. & Metcalfe, D. D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 125, S73–S80 (2010).
Acknowledgements
This work was funded by NIH grants AI175078, AI181815 and AI136920 to E.A.M. and AI175078-02S1 to F.W.S.
Author information
Authors and Affiliations
Contributions
F.W.S., Y.L., J.T. and E.A.M. contributed to the conception and writing of the paper. Editing, formatting and figure generation were performed by F.W.S. and E.A.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Trude Flo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Souza, F.W., Liu, Y., Trujillo, J. et al. Gasdermins against intracellular bacterial pathogens. Nat Microbiol (2026). https://doi.org/10.1038/s41564-026-02272-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41564-026-02272-z