Abstract
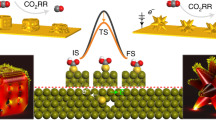
Highly active metal nanoparticles are desired to serve in high-temperature electrocatalysis, for example, in solid oxide electrochemical cells. Unfortunately, the low thermal stability of nanosized particles and the sophisticated interface requirement for electrode structures to support concurrent ionic and electronic transport make it hard to identify the exact catalytic role of nanoparticles embedded within complex electrode architectures. Here we present an accurate analysis of the reactivity of oxide electrodes boosted by metal nanoparticles, where all particles participate in the reaction. Monodisperse particles (Pt, Pd, Au and Co), 10 nm in size and stable at high temperature (more than 600 °C), are uniformly distributed onto mixed-conducting oxide electrodes as a model electrochemical cell via self-assembled nanopatterning. We identify how the metal catalysts activate hydrogen electrooxidation on the ceria-based electrode surface and quantify how rapidly the reaction rate increases with proper choice of metal. These results suggest an ideal electrode design for high-temperature electrochemical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Shao, Z. P. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Seo, H. G., Choi, Y. & Jung, W. Exceptionally enhanced electrode activity of (Pr,Ce)O2−δ-based cathodes for thin-film solid oxide fuel cells. https://doi.org/10.1002/aenm.201703647 (2018).
Gao, Z. et al. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 9, 1602–1644 (2016).
Irvine, J. T. S. et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 15014 (2016).
Vayssilov, G. N. et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 10, 310–315 (2011).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal–support interface role for ceria catalysts. Science 341, 771–773 (2013).
Lee, S., Seo, J. & Jung, W. Sintering-resistant Pt@CeO2 nanoparticles for high-temperature oxidation catalysis. Nanoscale 8, 10219–10228 (2016).
Cao, A., Lu, R. & Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 12, 13499–13510 (2010).
Cao, A. & Veser, G. Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles. Nat. Mater. 9, 75–81 (2010).
Gabaldon, J. P., Bore, M. & Datye, A. K. Mesoporous silica supports for improved thermal stability in supported Au catalysts. Top Catal. 44, 253–262 (2007).
Joo, S. H. et al. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 8, 126–131 (2009).
Prieto, G. et al. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 12, 34–39 (2013).
Arnal, P. M., Comotti, M. & Schuth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 45, 8224–8227 (2006).
Lu, J. L. et al. Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Science 335, 1205–1208 (2012).
Uchida, H., Suzuki, H. & Watanabe, M. High-performance electrode for medium-temperature solid oxide fuel cells—effects of composition and microstructures on performance of ceria-based anodes. J. Electrochem. Soc. 145, 615–620 (1998).
Jung, W., Gu, K. L., Choi, Y. & Haile, S. M. Robust nanostructures with exceptionally high electrochemical reaction activity for high temperature fuel cell electrodes. Energy Environ. Sci. 7, 1685–1692 (2014).
Kwak, N. W. et al. In situ synthesis of supported metal nanocatalysts through heterogeneous doping. Nat. Commun. 9, 4829 (2018).
Adijanto, L. et al. Synthesis and stability of Pd@CeO2 core–shell catalyst films in solid oxide fuel cell anodes. ACS Catal. 3, 1801–1809 (2013).
Zhu, Y. L. et al. Promotion of oxygen reduction by exsolved silver nanoparticles on a perovskite scaffold for low-temperature solid oxide duel xells. Nano Lett. 16, 512–518 (2016).
Kwon, O. et al. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 8, 15967 (2017).
Kwon, O. et al. Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells. J. Mater. Chem. A 6, 15947–15953 (2018).
Jiang, S. P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: advances and challenges. Int. J. Hydrogen Energy 37, 449–470 (2012).
Connor, P. A. et al. Tailoring SOFC electrode microstructures for improved performance. Adv. Energy Mater. 8, 1800120 (2018).
Myung, J. H., Neagu, D., Miller, D. N. & Irvine, J. T. S. Switching on electrocatalytic activity in solid oxide cells. Nature 537, 528–531 (2016).
Lai, W. & Haile, S. M. Impedance spectroscopy as a tool for chemical and electrochemical analysis of mixed conductors: a case study of ceria. J. Am. Ceram. Soc. 88, 2979–2997 (2005).
Chueh, W. C., Hao, Y., Jung, W. & Haile, S. M. High electrochemical activity of the oxide phase in model ceria–Pt and ceria–Ni composite anodes. Nat. Mater. 11, 155–161 (2012).
Choi, Y., Brown, E. C., Haile, S. M. & Jung, W. Electrochemically modified, robust solid oxide fuel cell anode for direct-hydrocarbon utilization. Nano Energy 23, 161–171 (2016).
Mun, J. H. et al. Monodisperse pattern nanoalloying for synergistic intermetallic catalysis. Nano Lett. 13, 5720–5726 (2013).
Cha, S. K. et al. Au–Ag core–shell nanoparticle array by block copolymer lithography for synergistic broadband plasmonic properties. ACS Nano 9, 5536–5543 (2015).
Polezhaeva, O. S., Yaroshinskaya, N. V. & Ivanov, V. K. Formation mechanism of nanocrystalline ceria in aqueous solutions of cerium(iii) nitrate and hexamethylenetetramine. Inorg. Mater. 44, 51–57 (2008).
Shinjoh, H. Noble metal sintering suppression technology in three-way catalyst: automotive three-way catalysts with the noble metal sintering suppression technology based on the support anchoring effect. Catal. Surv. Asia 13, 184–190 (2009).
Chen, C., Chen, D., Chueh, W. C. & Ciucci, F. Modeling the impedance response of mixed-conducting thin film electrodes. Phys. Chem. Chem. Phys. 16, 11573–11583 (2014).
Wang, X., Liu, D. P., Song, S. Y. & Zhang, H. J. Pt@CeO2 multicore@shell self-assembled nanospheres: clean synthesis, structure optimization, and catalytic applications. J. Am. Chem. Soc. 135, 15864–15872 (2013).
Bruix, A. et al. A new type of strong metal–support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J. Am. Chem. Soc. 134, 8968–8974 (2012).
Campbell, C. T. Catalyst–support interactions: electronic perturbations. Nat. Chem. 4, 597–598 (2012).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016).
Jung, W. et al. High electrode activity of nanostructured, columnar ceria films for solid oxide fuel cells. Energy Environ. Sci. 5, 8682–8689 (2012).
Kim, H. Y. & Henkelman, G. CO oxidation at the interface of Au nanoclusters and the stepped-CeO2 (111) surface by the Mars–van Krevelen mechanism. J. Phys. Chem. Lett. 4, 216–221 (2013).
Ha, H., Yoon, S., An, K. & Kim, H. Y. Catalytic CO oxidation over Au nanoparticles supported on CeO2 nanocrystals: effect of the Au–CeO2 interface. ACS Catal. 8, 11491–11501 (2018).
Zhang, C. J. et al. Mechanistic studies of water electrolysis and hydrogen electro-oxidation on high temperature ceria-based solid oxide electrochemical cells. J. Am. Chem. Soc. 135, 11572–11579 (2013).
Feng, Z. A. et al. Fast vacancy-mediated oxygen ion incorporation across the ceria–gas electrochemical interface. Nat. Commun. 5, 4374 (2014).
Lykhach, Y. et al. Oxide-based nanomaterials for fuel cell catalysis: the interplay between supported single Pt atoms and particles. Catal. Sci. Technol. 7, 4315–4345 (2017).
Karim, W. et al. Catalyst support effects on hydrogen spillover. Nature 541, 68–71 (2017).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L. et al. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Ismail, A., Giorgi, J. B. & Woo, T. K. On the atomistic interactions that direct ion conductivity and defect segregation in the bulk and surface of samarium-doped ceria: a genetic algorithm study. J. Phys. Chem. C 116, 704–713 (2012).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Acknowledgements
Y.C., S.L. and W.J. were supported financially by the Nano·Material Technology Development Program (NRF-2017M3A7B4049507), the Global Frontier R&D Program (2011-0031569) and the Basic Research Program (2014R1A4A1003712) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning. S.K.C. and S.O.K. were supported financially by the National Creative Research Initiative (CRI) Center for Multi-Dimensional Directed Nanoscale Assembly (2015R1A3A2033061) funded by NRF. H.H. and H.Y.K. acknowledge financial support from National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIP) (2017R1A2B4009829 and 2017R1A4A1015360). H.K.S. and J.Y.L. were supported financially by the Institute for Basic Science (IBS-R004-D1). This research used resources of the Center for Functional Nanomaterials, which is a US DOE Office of Science Facility, and the Scientific Data and Computing Center, a part of the Computational Science Initiative at Brookhaven National Laboratory (contract no. DE-SC0012704).
Author information
Authors and Affiliations
Contributions
Y.C., S.L. and W.J. conceived the idea for this study. Y.C. prepared the electrode cells and collected the electrocatalytic data. S.K.C. synthesized metal nanoparticles using BCP. H.H. performed DFT calculations. S.L. performed the CO oxidation test. H.K.S. and J.Y.L. performed TEM characterization. W.J., S.O.K. and H.Y.K. supervised the project and wrote the manuscript. All authors commented on the data and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Discussions, Figures 1–12, Table 1–2 and References.
Rights and permissions
About this article
Cite this article
Choi, Y., Cha, S.K., Ha, H. et al. Unravelling inherent electrocatalysis of mixed-conducting oxide activated by metal nanoparticle for fuel cell electrodes. Nat. Nanotechnol. 14, 245–251 (2019). https://doi.org/10.1038/s41565-019-0367-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-019-0367-4