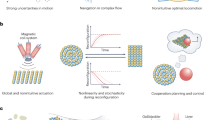
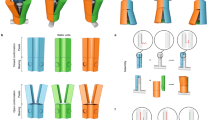
Abstract
Since their discovery in 2004, there has been remarkable progress in research on nanomotors, from the elucidation of different propulsion mechanisms to the study of their collective behaviour, culminating in investigations into their applications in biomedicine and environmental remediation. This Perspective reviews this evolution in nanomotor research and discusses the key challenges ahead, including the need for developing advanced characterization techniques, precise motion control, materials innovation, theory and modelling, and translationally feasible in vivo biomedical applications. These challenges highlight the current limitations of synthetic nanomotors and point to exciting future opportunities to revolutionize theranostics and create ‘living’ hybrid systems. We introduce the concept of ‘systems materials’ to encompass interacting functional materials across length scales from molecular to macro. Thus, this Perspective aims to inspire future generations of researchers to advance both fundamental understanding and practical breakthroughs, thereby engineering a paradigm shift in nanomotor research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Paxton, W. F. et al. Catalytic nanomotors: autonomous movement of striped nanorods. J. Am. Chem. Soc. 126, 13424–13431 (2004). A landmark study that introduced chemically powered nanomotors, launching the field of synthetic nanomotor research.
Fournier-Bidoz, S., Arsenault, A. C., Manners, I. & Ozin, G. A. Synthetic self-propelled nanorotors. Chem. Commun. 41, 441–443 (2005).
Yesin, K. B., Vollmers, K. & Nelson, B. J. Analysis and design of wireless magnetically guided microrobots in body fluids. Proc. IEEE Int. Conf. Robot. Autom. 2, 1333–1338 (2004).
Yesin, K. B., Vollmers, K. & Nelson, B. J. in Experimental Robotics IX. Springer Tracts in Advanced Robotics Vol. 21 (eds Ang, M. H. & Khatib, O.) 321–330 (Springer, 2006).
Golestanian, R., Liverpool, T. B. & Ajdari, A. Propulsion of a molecular machine by asymmetric distribution of reaction products. Phys. Rev. Lett. 94, 220801 (2005). This research article provides the foundational theoretical framework for self-diffusiophoresis as a propulsion mechanism for nanomotors.
Howse, J. R. et al. Self-motile colloidal particles: from directed propulsion to random walk. Phys. Rev. Lett. 99, 048102 (2007).
Solovev, A. A., Mei, Y., Ureña, E. B., Huang, G. & Schmidt, O. G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 5, 1688–1692 (2009).
Ibele, M., Mallouk, T. E. & Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 121, 3358–3362 (2009).
Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of light-activated colloidal surfers. Science 339, 933–936 (2013). An innovative research article highlighting light-powered active particles that form dynamic biomimetic self-assembled structures.
Dai, B. et al. Programmable artificial phototactic microswimmer. Nat. Nanotechnol. 11, 1087–1092 (2016). This work demonstrated phototactic behaviour in a group of synthetic microswimmers, mimicking the collective phototactic behaviour of green algae.
Yesin, K. B., Vollmers, K. & Nelson, B. J. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int. J. Robot. Res. 25, 527–536 (2006).
Bell, D. J., Leutenegger, S., Hammar, K. M., Dong, L. X. & Nelson, B. J. Flagella-like propulsion for microrobots using a nanocoil and a rotating electromagnetic field. Proc. IEEE Int. Conf. Robot. Autom. 24, 1128–1133 (2007).
Ghosh, A. & Fischer, P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 9, 2243–2245 (2009). A groundbreaking research article that demonstrated precise propulsion and control of magnetic nanomotors in fluidic environments.
Fan, D. et al. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires. Nat. Nanotechnol. 5, 545–551 (2010).
Kim, K., Xu, X., Guo, J. & Fan, D. L. Ultrahigh-speed rotating nanoelectromechanical system devices assembled from nanoscale building blocks. Nat. Commun. 5, 3632 (2014).
Wang, W., Castro, L. A., Hoyos, M. & Mallouk, T. E. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano 6, 6122–6132 (2012).
Wang, W. et al. Acoustic propulsion of nanorod motors inside living cells. Angew. Chem. Int. Ed. 53, 3201–3204 (2014). The demonstration of acoustic propulsion of nanorods inside living cells.
Ren, L. et al. 3D steerable, acoustically powered microswimmers for single-particle manipulation. Sci. Adv. 5, eaax3084 (2019).
Xu, T. et al. Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. J. Am. Chem. Soc. 137, 2163–2166 (2015).
Feng, J., Yuan, J. & Cho, S. K. Micropropulsion by an acoustic bubble for navigating microfluidic spaces. Lab Chip 15, 1554–1562 (2015).
Najafi, A. & Golestanian, R. Simple swimmer at low Reynolds number: three linked spheres. Phys. Rev. E 69, 062901–062904 (2004).
Dreyfus, R. et al. Microscopic artificial swimmers. Nature 437, 862–865 (2005).
Hong, Y., Blackman, N. M. K., Kopp, N. D., Sen, A. & Velegol, D. Chemotaxis of nonbiological colloidal rods. Phys. Rev. Lett. 99, 178103–178106 (2007).
Dey, K. K. et al. Chemotactic separation of enzymes. ACS Nano 8, 11941–11949 (2014).
Baraban, L., Harazim, S. M., Sanchez, S. & Schmidt, O. G. Chemotactic behavior of catalytic motors in microfluidic channels. Angew. Chem. Int. Ed. 52, 5552–5556 (2013).
Peng, F., Tu, Y., Van Hest, J. C. M. & Wilson, D. A. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew. Chem. Int. Ed. 54, 11662–11665 (2015).
Kagan, D., Balasubramanian, S. & Wang, J. Chemically triggered swarming of gold microparticles. Angew. Chem. Int. Ed. 123, 523–526 (2011).
Wang, W., Duan, W., Sen, A. & Mallouk, T. E. Catalytically powered dynamic assembly of rod-shaped nanomotors and passive tracer particles. Proc. Natl Acad. Sci. USA 110, 17744–17749 (2013).
Solovev, A. A., Sanchez, S. & Schmidt, O. G. Collective behaviour of self-propelled catalytic micromotors. Nanoscale 5, 1284–1293 (2013).
Yu, J. et al. Active generation and magnetic actuation of microrobotic swarms in bio-fluids. Nat. Commun. 10, 5631 (2019).
Xu, D. et al. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano 15, 11543–11554 (2021).
Altemose, A. et al. Chemically controlled spatiotemporal oscillations of colloidal assemblies. Angew. Chem. Int. Ed. 56, 7817–7821 (2017).
Singh, D. P., Choudhury, U., Fischer, P. & Mark, A. G. Non-equilibrium assembly of light-activated colloidal mixtures. Adv. Mater. 29, 1701328 (2017).
Walker, D., Käsdorf, B. T., Jeong, H. H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Ramos-Docampo, M. A. et al. Microswimmers with heat delivery capacity for 3D cell spheroid penetration. ACS Nano 13, 12192–12205 (2019).
Gardi, G., Ceron, S., Wang, W., Petersen, K. & Sitti, M. Microrobot collectives with reconfigurable morphologies, behaviors, and functions. Nat. Commun. 13, 2239 (2022).
Chen, S. et al. Collective buoyancy-driven dynamics in swarming enzymatic nanomotors. Nat. Commun. 15, 9315 (2024).
Ruiz-González, N. et al. Swarms of enzyme-powered nanomotors enhance the diffusion of macromolecules in viscous media. Small 20, 2309387–2309403 (2024).
Sun, M. et al. Individual and collective manipulation of multifunctional bimodal droplets in three dimensions. Sci. Adv. 10, eadp1439 (2024).
Patino, T. et al. Self-sensing enzyme-powered micromotors equipped with pH-responsive DNA nanoswitches. Nano Lett. 19, 3440–3447 (2019).
Liu, X. et al. Urease-powered micromotors with spatially selective distribution of enzymes for capturing and sensing exosomes. ACS Nano 17, 24343–24354 (2023).
Yuan, K., López, M. Á., Jurado-Sánchez, B. & Escarpa, A. Janus micromotors coated with 2D nanomaterials as dynamic interfaces for (bio)-sensing. ACS Appl. Mater. Interfaces 12, 46588–46597 (2020).
Li, H. et al. Precise electrokinetic position and three-dimensional orientation control of a nanowire bioprobe in solution. Nat. Nanotechnol. 18, 1213–1221 (2023).
Esteban-Fernández De Ávila, B. et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 10, 4997–5005 (2016).
Tu, Y. et al. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 11, 1957–1963 (2017).
Xu, H., Medina-Sánchez, M., Maitz, M. F., Werner, C. & Schmidt, O. G. Sperm micromotors for cargo delivery through flowing blood. ACS Nano 14, 2982–2993 (2020).
Hortelão, A. C., Patiño, T., Perez-Jiménez, A., Blanco, À. & Sánchez, S. Enzyme-powered nanobots enhance anticancer drug delivery. Adv. Funct. Mater. 28, 1705086 (2018).
Ma, X., Hahn, K. & Sanchez, S. Catalytic mesoporous Janus nanomotors for active cargo delivery. J. Am. Chem. Soc. 137, 4976–4979 (2015).
Solovev, A. A., Sanchez, S., Pumera, M., Mei, Y. F. & Schmidt, O. C. Magnetic control of tubular catalytic microbots for the transport, assembly, and delivery of micro-objects. Adv. Funct. Mater. 20, 2430–2435 (2010).
Wang, Q. et al. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 7, eabe5914 (2021).
Ye, Z. et al. Supramolecular modular assembly of imaging-trackable enzymatic nanomotors. Angew. Chem. Int. Ed. 63, e202401209 (2024).
Zheng, S. et al. Biocompatible nanomotors as active diagnostic imaging agents for enhanced magnetic resonance imaging of tumor tissues in vivo. Adv. Funct. Mater. 31, 2100936 (2021).
Vilela, D. et al. Medical imaging for the tracking of micromotors. ACS Nano 12, 1220–1227 (2018).
Hortelao, A. C. et al. Swarming behavior and in vivo monitoring of enzymatic nanomotors within the bladder. Sci. Robot. 6, eabd2823 (2021).
Wu, Z. et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 4, eaax0613 (2019).
Simó, C. et al. Urease-powered nanobots for radionuclide bladder cancer therapy. Nat. Nanotechnol. 19, 554–564 (2024). The therapeutic use of enzyme-powered nanomotors with radionuclide payloads in vivo, marking a translational milestone.
Chen, S. et al. Dual-source powered nanomotor with integrated functions for cancer photo-theranostics. Biomaterials 288, 121744–121753 (2022).
Yan, X. et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2, eaaq1155 (2017).
Soler, L., Magdanz, V., Fomin, V. M., Sanchez, S. & Schmidt, O. G. Self-propelled micromotors for cleaning polluted water. ACS Nano 7, 9611–9620 (2013). A demonstration of self-propelled micromotors for the efficient oxidation of organic pollutants by improving intermixing in liquids.
Orozco, J. et al. Artificial enzyme-powered microfish for water-quality testing. ACS Nano 7, 818–824 (2013).
Villa, K., Parmar, J., Vilela, D. & Sánchez, S. Metal-oxide-based microjets for the simultaneous removal of organic pollutants and heavy metals. ACS Appl. Mater. Interfaces 10, 20478–20486 (2018).
Villa, K. et al. Visible-light-driven single-component BiVO4 micromotors with the autonomous ability for capturing microorganisms. ACS Nano 13, 8135–8145 (2019).
Ye, H. et al. Atomic H* mediated fast decontamination of antibiotics by bubble-propelled magnetic iron-manganese oxides core-shell micromotors. Appl. Catal. B 314, 121484 (2022).
Chen, C., Ding, S. & Wang, J. Materials consideration for the design, fabrication and operation of microscale robots. Nat. Rev. Mater. 9, 159–172 (2024).
Wang, Y. et al. Swarm autonomy: from agent functionalization to machine intelligence. Adv. Mater. 37, 202312956 (2024).
Zhang, Y. & Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 5, 500–510 (2021).
Chen, S., Prado-Morales, C., Sánchez-DeAlcázar, D. & Sánchez, S. Enzymatic micro/nanomotors in biomedicine: from single motors to swarms. J. Mater. Chem. B 12, 2711–2719 (2024).
Wang, Q., Yang, S. & Zhang, L. Untethered micro/nanorobots for remote sensing: toward intelligent platform. Nano Micro Lett. 16, 40 (2024).
Dutta, S. et al. Recent developments in metallic degradable micromotors for biomedical and environmental remediation applications. Nano Micro Lett. 16, 1–35 (2023).
Yang, L. et al. Autonomous environment-adaptive microrobot swarm navigation enabled by deep learning-based real-time distribution planning. Nat. Mach. Intell. 4, 480–493 (2022). A breakthrough in microrobot swarm navigation by integrating machine learning for environment-adaptive reconfiguration, thereby connecting computational intelligence to microrobot swarming.
Ghosh, A. et al. Helical nanomachines as mobile viscometers. Adv. Funct. Mater. 28, 1705687 (2018).
Patiño, T., Llacer-Wintle, J., Pujals, S., Albertazzi, L. & Sánchez, S. Unveiling protein corona formation around self-propelled enzyme nanomotors by nanoscopy. Nanoscale 16, 2904–2912 (2023).
Dasgupta, D. et al. Mobile nanobots for prevention of root canal treatment failure. Adv. Healthc. Mater. 11, 2200232 (2022).
Dasgupta, D., Pally, D., Saini, D. K., Bhat, R. & Ghosh, A. Nanomotors sense local physicochemical heterogeneities in tumor microenvironments. Angew. Chem. Int. Ed. 59, 23690–23696 (2020).
Gao, C., Zhou, C., Lin, Z., Yang, M. & He, Q. Surface wettability-directed propulsion of glucose-powered nanoflask motors. ACS Nano 13, 12758–12766 (2019).
Simmchen, J. et al. Topographical pathways guide chemical microswimmers. Nat. Commun. 7, 10598 (2016).
Blanchard, A. T. et al. Highly polyvalent DNA motors generate 100+ pN of force via autochemophoresis. Nano Lett. 19, 6977–6986 (2019).
Ma, X. et al. Enzyme-powered hollow mesoporous Janus nanomotors. Nano Lett. 15, 7043–7050 (2015).
Patiño, T. et al. Influence of enzyme quantity and distribution on the self-propulsion of non-Janus urease-powered micromotors. J. Am. Chem. Soc. 140, 7896–7903 (2018).
Singh, D. P. et al. Interface-mediated spontaneous symmetry breaking and mutual communication between drops containing chemically active particles. Nat. Commun. 11, 2210 (2020).
Venugopalan, P. L. et al. Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 14, 1968–1975 (2014).
Venugopalan, P. L., Jain, S., Shivashankar, S. & Ghosh, A. Single coating of zinc ferrite renders magnetic nanomotors therapeutic and stable against agglomeration. Nanoscale 10, 2327–2332 (2018).
Zhang, L. et al. Artificial bacterial flagella: fabrication and magnetic control. Appl. Phys. Lett. 94, 064107 (2009).
Kadiri, V. M. et al. Biocompatible magnetic micro- and nanodevices: fabrication of FePt nanopropellers and cell transfection. Adv. Mater. 32, 2001114 (2020).
Peter, F. et al. Degradable and biocompatible magnesium zinc structures for nanomedicine: magnetically actuated liposome microcarriers with tunable release. Adv. Funct. Mater. 34, 2314265 (2024).
Wilson, D., Nolte, R. & van Hest, J. Autonomous movement of platinum-loaded stomatocytes. Nat. Chem. 4, 268–274 (2012).
Liang, Z. & Fan, D. Visible light-gated reconfigurable rotary actuation of electric nanomotors. Sci. Adv. 4, eaau0981 (2018).
Liang, Z., Teal, D. & Fan, D. Light programmable micro/nanomotors with optically tunable in-phase electric polarization. Nat. Commun. 10, 5275 (2019).
Liang, Z., Joh, H., Lian, B., Fan, D. E. & Fan, D. E. Light-stimulated micromotor swarms in an electric field with accurate spatial, temporal, and mode control. Sci. Adv. 9, eadi9932 (2023).
Zhang, J. et al. Light-powered, fuel-free oscillation, migration, and reversible manipulation of multiple cargo types by micromotor swarms. ACS Nano 17, 251–262 (2023).
Li, W. et al. Arbitrary construction of versatile NIR-driven microrobots. Adv. Mater. 36, 2402482 (2024).
Crosby, G. A., Watts, R. J. & Carstens, D. H. W. Inversion of excited states of transition-metal complexes. Science 170, 1195–1196 (1970).
Zhou, J., Liu, Q., Feng, W., Sun, Y. & Li, F. Upconversion luminescent materials: advances and applications. Chem. Rev. 115, 395–465 (2015).
Dey, K. K. et al. Micromotors powered by enzyme catalysis. Nano Lett. 15, 8311–8315 (2015).
Pantarotto, D., Browne, W. R. & Feringa, B. L. Autonomous propulsion of carbon nanotubes powered by a multienzyme ensemble. Chem. Commun. 44, 1533–1535 (2008).
Somasundar, A. et al. Positive and negative chemotaxis of enzyme-coated liposome motors. Nat. Nanotechnol. 14, 1129–1134 (2019).
Joseph, A. et al. Chemotactic synthetic vesicles: design and applications in blood-brain barrier crossing. Sci. Adv. 3, e1700362 (2017).
Agudo-Canalejo, J., Illien, P. & Golestanian, R. Phoresis and enhanced diffusion compete in enzyme chemotaxis. Nano Lett. 18, 2711–2717 (2018).
Zhao, X. et al. Substrate-driven chemotactic assembly in an enzyme cascade. Nat. Chem. 10, 311–317 (2018).
Arqué, X. et al. Intrinsic enzymatic properties modulate the self-propulsion of micromotors. Nat. Commun. 10, 2826 (2019).
Pumm, A. K. et al. A DNA origami rotary ratchet motor. Nature 607, 492–498 (2022).
Tran, M. P. et al. Genetic encoding and expression of RNA origami cytoskeletons in synthetic cells. Nat. Nanotechnol. 20, 664–671 (2025).
Yang, L., Yu, J. & Zhang, L. Statistics-based automated control for a swarm of paramagnetic nanoparticles in 2-D space. IEEE Trans. Robot. 36, 254–270 (2020).
Rückner, G. & Kapral, R. Chemically powered nanodimers. Phys. Rev. Lett. 98, 150603 (2007).
Thakur, S., Chen, J. X. & Kapral, R. Interaction of a chemically propelled nanomotor with a chemical wave. Angew. Chem. Int. Ed. 50, 10165–10169 (2011).
De Corato, M. et al. Self-propulsion of active colloids via ion release: theory and experiments. Phys. Rev. Lett. 124, 108001 (2020).
Golestanian, R. Anomalous diffusion of symmetric and asymmetric active colloids. Phys. Rev. Lett. 102, 188305 (2009).
Liebchen, B., Marenduzzo, D., Pagonabarraga, I. & Cates, M. E. Clustering and pattern formation in chemorepulsive active colloids. Phys. Rev. Lett. 115, 258301 (2015).
Das, S. et al. Boundaries can steer active Janus spheres. Nat. Commun. 6, 8999 (2015).
Palacios, L. S. et al. Guided accumulation of active particles by topological design of a second-order skin effect. Nat. Commun. 12, 4691 (2021).
Saha, S., Ramaswamy, S. & Golestanian, R. Pairing, waltzing and scattering of chemotactic active colloids. New J. Phys. 21, 063006 (2019).
Meredith, C. H. et al. Predator–prey interactions between droplets driven by non-reciprocal oil exchange. Nat. Chem. 12, 1136–1142 (2020).
Soto, R. & Golestanian, R. Self-assembly of catalytically active colloidal molecules: tailoring activity through surface chemistry. Phys. Rev. Lett. 112, 068301 (2014).
Mandal, N. S., Sen, A. & Astumian, R. D. A molecular origin of non-reciprocal interactions between interacting active catalysts. Chem 10, 1147–1159 (2024).
Tucci, G. et al. Nonreciprocal collective dynamics in a mixture of phoretic Janus colloids. New J. Phys. 26, 073006 (2024).
Agudo-Canalejo, J. & Golestanian, R. Active phase separation in mixtures of chemically interacting particles. Phys. Rev. Lett. 123, 018101 (2019).
Golestanian, R. in Active Matter and Nonequilibrium Statistical Physics: Lecture Notes of the Les Houches Summer School Vol. 112 (eds Tailleur, J. et al.) 230–293 (Oxford Academic, 2022).
Wang, Q. et al. Tracking and navigation of a microswarm under laser speckle contrast imaging for targeted delivery. Sci. Robot. 9, eadh1978 (2024).
Jin, D. et al. Swarming self-adhesive microgels enabled aneurysm on-demand embolization in physiological blood flow. Sci. Adv. 9, eadf9278 (2023).
Ahmed, D. et al. Bioinspired acousto-magnetic microswarm robots with upstream motility. Nat. Mach. Intell. 3, 116–124 (2021).
Palacci, J. et al. Artificial rheotaxis. Sci. Adv. 1, e1400214 (2015).
Ren, L. et al. Rheotaxis of bimetallic micromotors driven by chemical-acoustic hybrid power. ACS Nano 11, 10591–10598 (2017).
Choi, H., Cho, S. H. & Hahn, S. K. Urease-powered polydopamine nanomotors for intravesical therapy of bladder diseases. ACS Nano 14, 6683–6692 (2020).
Wu, Z. et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 4, eaat4388 (2018).
Xu, C. et al. Magnesium-based micromotors as hydrogen generators for precise rheumatoid arthritis therapy. Nano Lett. 21, 1982–1991 (2021).
Zhang, F. et al. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci. Adv. 10, eadn6157 (2024).
Arqué, X. et al. Autonomous treatment of bacterial infections in vivo using antimicrobial micro- and nanomotors. ACS Nano 16, 7547–7558 (2022).
Ji, X. et al. Multifunctional parachute-like nanomotors for enhanced skin penetration and synergistic antifungal therapy. ACS Nano 15, 14218–14228 (2021).
Dey, K. K. Dynamic coupling at low Reynolds number. Angew. Chem. Int. Ed. 58, 2208–2228 (2019).
Maiti, A., Koyano, Y., Kitahata, H. & Dey, K. K. Activity-induced diffusion recovery in crowded colloidal suspensions. Phys. Rev. E 109, 054607 (2024).
Pal, M. et al. Maneuverability of magnetic nanomotors inside living cells. Adv. Mater. 30, 1800429 (2018).
Aghakhani, A. et al. High shear rate propulsion of acoustic microrobots in complex biological fluids. Sci. Adv. 8, eabm5126 (2022).
Osat, S. & Golestanian, R. Non-reciprocal multifarious self-organization. Nat. Nanotechnol. 18, 79–85 (2022).
Manna, R. K., Gentile, K., Shklyaev, O. E., Sen, A. & Balazs, A. C. Self-generated convective flows enhance the rates of chemical reactions. Langmuir 38, 1432–1439 (2022).
Ma, X., Wang, X., Hahn, K. & Sánchez, S. Motion control of urea-powered biocompatible hollow microcapsules. ACS Nano 10, 3597–3605 (2016).
Mehta, P., Lang, A. H. & Schwab, D. J. Landauer in the age of synthetic biology: energy consumption and information processing in biochemical networks. J. Stat. Phys. 162, 1153–1166 (2016).
Jahnke, K. et al. DNA origami signaling units transduce chemical and mechanical signals in synthetic cells. Adv. Funct. Mater. 34, 2301176 (2024).
Zhang, F. et al. ACE2 receptor-modified algae-based microrobot for removal of SARS-CoV-2 in wastewater. J. Am. Chem. Soc. 143, 12194–12201 (2021).
Yuan, X., Ferrer-Campos, R., Garcés-Pineda, F. A. & Villa, K. Molecular imprinted BiVO4 microswimmers for selective target recognition and removal. Small 19, 2207303 (2023).
Yuan, X. et al. Self-degradable photoactive micromotors for inactivation of resistant bacteria. Adv. Opt. Mater. 12, 2303137 (2024).
Guix, M. et al. Superhydrophobic alkanethiol-coated microsubmarines for effective removal of oil. ACS Nano 6, 4445–4451 (2012).
Ferrer Campos, R., Bachimanchi, H., Volpe, G. & Villa, K. Bubble-propelled micromotors for ammonia generation. Nanoscale 15, 15785–15793 (2023).
Ferrer Campos, R. et al. Boosting the efficiency of photoactive rod-shaped nanomotors via magnetic field-induced charge separation. ACS Appl. Mater. Interfaces 16, 30077–30087 (2024).
Parmar, J. et al. Reusable and long-lasting active microcleaners for heterogeneous water remediation. Adv. Funct. Mater. 26, 4152–4161 (2016).
Vilela, D., Guix, M., Parmar, J., Blanco-Blanes, À. & Sánchez, S. Micromotor-in-sponge platform for multicycle large-volume degradation of organic pollutants. Small 18, 2107619 (2022).
Zhang, S. et al. 3D-printed micrometer-scale wireless magnetic cilia with metachronal programmability. Sci. Adv. 9, eadf9462 (2023).
Tottori, S. et al. Magnetic helical micromachines: fabrication, controlled swimming, and cargo transport. Adv. Mater. 24, 811–816 (2012). The demonstration of two-photon laser-printed microrobots.
Hsu, L. Y. et al. Alignment and actuation of liquid crystals via 3D confinement and two-photon laser printing. Sci. Adv. 10, 2597 (2024).
Melde, K. et al. Ultrasound-assisted tissue engineering. Nat. Rev. Bioeng. 2, 486–500 (2024).
Kriebisch, C. M. E. et al. A roadmap toward the synthesis of life. Chem 11, 102399 (2025).
Balazs, A. C., Fischer, P. & Sen, A. Intelligent nano/micromotors: using free energy to fabricate organized systems driven far from equilibrium. Acc. Chem. Res. 51, 2979 (2018).
Song, J., Shklyaev, O. E., Sapre, A., Balazs, A. C. & Sen, A. Self-propelling macroscale sheets powered by enzyme pumps. Angew. Chem. Int. Ed. 136, e202311556 (2024).
Gao, W. et al. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9, 117–123 (2015).
Li, J. et al. Enteric micromotor can selectively position and spontaneously propel in the gastrointestinal tract. ACS Nano 10, 9536–9542 (2016).
De Ávila, B. E. F. et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 8, 272 (2017).
Zhang, F. et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 21, 1324–1332 (2022). Biohybrid microrobots for the active delivery of antibiotics in the lungs in vivo, demonstrating notable potential for clinical applications in intensive care units.
Su, L. et al. Modularized microrobot with lock-and-detachable modules for targeted cell delivery in bile duct. Sci. Adv. 9, eadj0883 (2023).
Feng, Y. et al. Directed neural stem cells differentiation via signal communication with Ni–Zn micromotors. Adv. Mater. 35, 2301736 (2023).
Choi, H. et al. Urease-powered nanomotor containing STING agonist for bladder cancer immunotherapy. Nat. Commun. 15, 9934 (2024).
Gao, C. et al. Light-driven artificial cell micromotors for degenerative knee osteoarthritis. Adv. Mater. 37, 2416349 (2025).
Kagan, D. et al. Chemical sensing based on catalytic nanomotors: motion-based detection of trace silver. J. Am. Chem. Soc. 131, 12082–12083 (2009).
Campuzano, S. et al. Bacterial isolation by lectin-modified microengines. Nano Lett. 12, 396–401 (2012).
Li, J. et al. Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents. ACS Nano 8, 11118–11125 (2014).
Gao, L., Giglio, K. M., Nelson, J. L., Sondermann, H. & Travis, A. J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 6, 2588–2593 (2014).
Vilela, D., Parmar, J., Zeng, Y., Zhao, Y. & Sánchez, S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 16, 2860–2866 (2016).
Jurado-Sánchez, B., Pacheco, M., Rojo, J. & Escarpa, A. Magnetocatalytic graphene quantum dots Janus micromotors for bacterial endotoxin detection. Angew. Chem. Int. Ed. 56, 6957–6961 (2017).
Villa, K., Děkanovský, L., Plutnar, J., Kosina, J. & Pumera, M. Swarming of perovskite-like Bi2WO6 microrobots destroy textile fibers under visible light. Adv. Funct. Mater. 30, 2007073 (2020).
Mou, F. et al. ZnO-based micromotors fueled by CO2: the first example of self-reorientation-induced biomimetic chemotaxis. Natl Sci. Rev. 8, nwab066 (2021).
Urso, M., Ussia, M., Novotný, F. & Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 13, 3573(2022).
Patiño, T. et al. Synthetic DNA-based swimmers driven by enzyme catalysis. J. Am. Chem. Soc. 146, 12664–12671 (2024).
Acknowledgements
S.S. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 and Horizon Europe research and innovation programmes (grant agreement numbers 866348, i-NanoSwarms, and 101138723, MucOncoBots, 101189423, OrthoBots) and from ‘la Caixa’ Foundation under the grant agreement LCF/PR/HR21/52410022. A.S. thanks S. Sen for many stimulating discussions. A.S. also acknowledges funding by the National Science Foundation, the Air Force Office of Scientific Research, the Defense Threat Reduction Agency, the Charles E. Kaufman Foundation and the Alfred P. Sloan Foundation. J.T. thanks the funding support from the Hong Kong Research Grants Council (RGC) (C7082-21G). L.Z. thanks funding support from the Hong Kong Research Grants Council RGC (R4015-2, RFS2122-4S03, STG1/E-401/23-N). W.W. thanks the National Natural Science Foundation of China (grant number T2322006). K.V. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (GA number 101076680; PhotoSwim) and the grant PID2022-136886OA-I00 financed by MCIN/AEI/10.13039/501100011033/ FEDER, UE. H.H. gratefully acknowledges support from NSF grant 2230116 and ARO grant W911NF-22-1-0047. R.G. acknowledges support from the Max Planck School Matter to Life and the MaxSynBio Consortium, which are jointly funded by the Federal Ministry of Education and Research (BMBF) of Germany and the Max Planck Society. A.G. thanks the Wellcome Trust/DBT India Alliance Fellowships/Grants (grant number IA/S/19/2/504655). D.E.F. thanks the support of the National Science Foundation (2219221 and 1930649). S.C. acknowledges the Predoctoral AGAUR-FI Joan Oró grant (2023 FI-1 00654) funded by ‘Secretaria d’Universitats i Recerca del Departament de Recerca i Universitats de la Generalitat de Catalunya’ and by European Social Fund Plus.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Motilal Mathesh and Fei Peng for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Fan, D.E., Fischer, P. et al. A roadmap for next-generation nanomotors. Nat. Nanotechnol. 20, 990–1000 (2025). https://doi.org/10.1038/s41565-025-01962-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01962-9