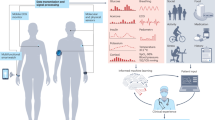
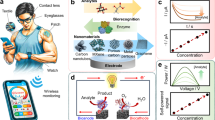
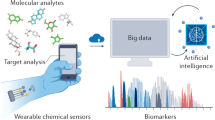
Abstract
Over the past decade, consumer wearable sensors have become increasingly ubiquitous in health monitoring, enabling the widespread tracking of key biophysical parameters. The transition towards next-generation body-interfaced biomolecular sensing technologies, fuelled by the integration of reagentless sensing strategies with advanced nanomaterials, marks the next substantial leap forward. These innovations enable unobtrusive, multimodal monitoring of both physiological parameters and biochemical disease markers in real time. This Review examines the current generation of body-interfaced biomolecular sensing technologies, with a particular emphasis on materials innovation and nanotechnological advancements, and discusses their pivotal role in chronic disease monitoring. The discussion extends to the challenges and prospects in this rapidly evolving field, highlighting the potential for materials-focused approaches to transform the landscape of chronic disease monitoring and management with body-interfaced bioelectronics. By harnessing the power of materials and nanotechnological innovations, these biomolecular sensing technologies promise to enhance diagnostic capabilities and foster a more proactive, personalized approach to combating these diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
The top 10 causes of death. WHO https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Zhao, C., Park, J., Root, S. E. & Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2, 671–690 (2024).
Lee, G.-H. et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 5, 149–165 (2020).
Flynn, C. D. et al. Biomolecular sensors for advanced physiological monitoring. Nat. Rev. Bioeng. 1, 560–575 (2023).
Townsend, N. et al. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 19, 133–143 (2022).
Mahmud, A. et al. Monitoring cardiac biomarkers with aptamer-based molecular pendulum sensors. Angew. Chem. Int. Ed. 62, e202213567 (2023).
Saenger, A. K. A tale of two biomarkers: the use of troponin and CK-MB in contemporary practice. Am. Soc. Clin. Lab. Sci. 23, 134–140 (2010).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Prim. 5, 56 (2019).
Wang, H., Rosendaal, F. R., Cushman, M. & Van Hylckama Vlieg, A. D‐dimer, thrombin generation, and risk of a first venous thrombosis in the elderly. Res. Pract. Thromb. Haemost. 5, e12536 (2021).
Pan, X. et al. Associations of circulating choline and its related metabolites with cardiometabolic biomarkers: an international pooled analysis. Am. J. Clin. Nutr. 114, 893–906 (2021).
Tousoulis, D. et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int. J. Cardiol. 167, 1924–1928 (2013).
Tomic, D., Shaw, J. E. & Magliano, D. J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 18, 525–539 (2022).
Lee, M. et al. β-hydroxybutyrate as a biomarker of β-cell function in new-onset type 2 diabetes and its association with treatment response at 6 months. Diabetes Metab. 49, 101427 (2023).
Zheng, X. et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat. Commun. 12, 1487 (2021).
Katsiki, N., Mikhailidis, D. P. & Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 39, 1176–1188 (2018).
Theofilopoulos, A. N., Kono, D. H. & Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 18, 716–724 (2017).
Kolarz, B., Podgorska, D. & Podgorski, R. Insights of rheumatoid arthritis biomarkers. Biomarkers 26, 185–195 (2021).
Pisetsky, D. S. Anti-DNA antibodies—quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 12, 102–110 (2016).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Colhoun, H. M. & Marcovecchio, M. L. Biomarkers of diabetic kidney disease. Diabetologia 61, 996–1011 (2018).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589 (2018).
Hagberg, L., Edén, A., Zetterberg, H., Price, R. W. & Gisslén, M. Blood biomarkers for HIV infection with focus on neurologic complications—a review. Acta Neurol. Scand. 146, 56–60 (2022).
Busche, M. A. & Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23, 1183–1193 (2020).
Emin, D. et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat. Commun. 13, 5512 (2022).
Sarkar, S. et al. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 1646, 139–151 (2016).
Martinez, B. & Peplow, P. MicroRNAs in Parkinson’s disease and emerging therapeutic targets. Neural Regen. Res. 12, 1945–1959 (2017).
Otani, N., Hoshiyama, E., Ouchi, M., Takekawa, H. & Suzuki, K. Uric acid and neurological disease: a narrative review. Front. Neurol. 14, 1164756 (2023).
Moustafa, A. A., Hewedi, D. H., Eissa, A. M., Frydecka, D. & Misiak, B. in Diet and Exercise in Cognitive Function and Neurological Diseases (eds. Farooqui, T. & Farooqui, A. A.) 73–81 (Wiley, 2015).
Wang, L., Hu, Y., Jiang, N. & Yetisen, A. K. Biosensors for psychiatric biomarkers in mental health monitoring. Biosens. Bioelectron. 256, 116242 (2024).
Zamani, M., Wilhelm, T. & Furst, A. L. Perspective—electrochemical sensors for neurotransmitters and psychiatrics: steps toward physiological mental health monitoring. J. Electrochem. Soc. 169, 047513 (2022).
Schumann, G. et al. Stratified medicine for mental disorders. Eur. Neuropsychopharmacol. 24, 5–50 (2014).
Rutsch, A., Kantsjö, J. B. & Ronchi, F. The gut–brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11, 604179 (2020).
Roomruangwong, C. et al. Menstruation distress is strongly associated with hormone–immune–metabolic biomarkers. J. Psychosom. Res. 142, 110355 (2021).
Ciebiera, M. et al. Nutrition in gynecological diseases: current perspectives. Nutrients 13, 1178 (2021).
Mavreli, D., Theodora, M. & Kolialexi, A. Known biomarkers for monitoring pregnancy complications. Expert Rev. Mol. Diagn. 21, 1115–1117 (2021).
Eastell, R. & Hannon, R. A. Biomarkers of bone health and osteoporosis risk. Proc. Nutr. Soc. 67, 157–162 (2008).
Amin, M. N. et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 8, 205031212096575 (2020).
Jain, K. K. in The Handbook of Biomarkers 27–238 (Springer, 2017).
Liu, C. H. et al. Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nat. Immunol. 18, 1175–1180 (2017).
Tu, J. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 7, 1293–1306 (2023). This paper introduces a wearable, wireless patch that enables real-time, non-invasive monitoring of the inflammatory biomarker C-reactive protein in sweat, correlating with serum levels and demonstrating high translation potential for the point-of-care management of chronic diseases.
Liu, Y. et al. Revolutionizing precision medicine: exploring wearable sensors for therapeutic drug monitoring and personalized therapy. Biosensors 13, 726 (2023).
Sempionatto, J. R., Montiel, V. R.-V., Vargas, E., Teymourian, H. & Wang, J. Wearable and mobile sensors for personalized nutrition. ACS Sens. 6, 1745–1760 (2021).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022). This study reports on a wearable electrochemical biosensor capable of continuously monitoring multiple non-electroactive metabolites and nutrients, including all essential amino acids and vitamins, in sweat during both exercise and rest, utilizing innovative MIPs, redox-active nanoparticles and integrated sweat induction and sampling technologies.
Güttler, N. et al. Omega-3 fatty acids and vitamin D in cardiology. Cardiol. Res. Pract. 2012, 729670 (2012).
Kevadiya, B. D. et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 20, 593–605 (2021).
Wang, C. et al. Wound management materials and technologies from bench to bedside and beyond. Nat. Rev. Mater. 9, 550–566 (2024).
Cappon, G., Vettoretti, M., Sparacino, G. & Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab. J. 43, 383–397 (2019).
Arlett, J. L., Myers, E. B. & Roukes, M. L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 6, 203–215 (2011).
Zhang, Y. et al. Nanozymes for nanohealthcare. Nat. Rev. Methods Prim. 4, 36 (2024).
Wu, X., Ge, J., Yang, C., Hou, M. & Liu, Z. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment. Chem. Commun. 51, 13408–13411 (2015).
Wang, M. et al. Printable molecule-selective core–shell nanoparticles for wearable and implantable sensing. Nat. Mater. 24, 589–598 (2025). This study reports on a skin-interfaced, printable wearable sensor leveraging core–shell nanoparticles with MIP shells for customizable and selective sweat analysis, featuring electrochemical regeneration via electrical pulses for repeated use without performance loss.
Yao, J., Yang, M. & Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: new insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 114, 6130–6178 (2014).
Biswas, A. et al. Advances in top-down and bottom-up surface nanofabrication: techniques, applications & future prospects. Adv. Colloid Interface Sci. 170, 2–27 (2012).
Wang, P. et al. DNA origami guided self-assembly of plasmonic polymers with robust long-range plasmonic resonance. Nano Lett. 20, 8926–8932 (2020).
Fu, K. et al. Accelerated electron transfer in nanostructured electrodes improves the sensitivity of electrochemical biosensors. Adv. Sci. 8, 2102495 (2021).
Altug, H., Oh, S.-H., Maier, S. A. & Homola, J. Advances and applications of nanophotonic biosensors. Nat. Nanotechnol. 17, 5–16 (2022).
Bauch, M., Toma, K., Toma, M., Zhang, Q. & Dostalek, J. Plasmon-enhanced fluorescence biosensors: a review. Plasmonics 9, 781–799 (2014).
Fu, W. et al. Efficient optical plasmonic tweezer-controlled single-molecule SERS characterization of pH-dependent amylin species in aqueous milieus. Nat. Commun. 14, 6996 (2023).
Min, J. et al. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123, 5049–5138 (2023).
Xu, Y. et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 7, 1307–1320 (2023).
Arwani, R. T. et al. Stretchable ionic–electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 23, 1115–1122 (2024). This article reports on a stretchable ionic–electronic bilayer hydrogel sensor capable of detecting solid-state epidermal biomarkers, including cholesterol and lactate, directly on the skin surface without the need for fluid sampling, combining innovative materials science with electrochemical sensing for non-invasive, continuous health monitoring.
Ye, C. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 19, 330–337 (2024).
Wu, Z. et al. Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications. Commun. Mater. 5, 33 (2024).
Friedel, M. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 7, 1541–1555 (2023).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022). In this study, multiple biomarkers were continuously monitored using a fully integrated wearable microneedle array in ISF during daily activities.
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021).
Xiong, Z. et al. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci. Adv. 7, eabj1617 (2021).
Lee, Y. et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl Acad. Sci. USA 115, 5377–5382 (2018).
Mannoor, M. S. et al. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 3, 763 (2012).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Güntner, A. T. et al. Breath sensors for health monitoring. ACS Sens. 4, 268–280 (2019).
Ates, H. C. & Dincer, C. Wearable breath analysis. Nat. Rev. Bioeng. 1, 80–82 (2023).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 39, 1366–1374 (2021). In this study, freeze-dried, cell-free synthetic biology circuits were successfully integrated into wearable materials, enabling the non-invasive detection of various biomolecules, chemicals and pathogens, including SARS-CoV-2, without the need for living cells or complex instrumentation.
Heng, W. et al. A smart mask for exhaled breath condensate harvesting and analysis. Science 385, 954–961 (2024). This work demonstrates continuous breath condensate sampling and accurate electrochemical monitoring of metabolites and inflammatory biomarkers in exhaled breath, offering practical applications in both daily life and clinical settings.
Ye, Y. et al. Smart contact lens with dual-sensing platform for monitoring intraocular pressure and matrix metalloproteinase-9. Adv. Sci. 9, 2104738 (2022).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Keum, D. H. et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6, eaba3252 (2020).
Sempionatto, J. R. et al. Eyeglasses-based tear biosensing system: non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 137, 161–170 (2019).
Seo, H. et al. Smart contact lenses as wearable ophthalmic devices for disease monitoring and health management. Chem. Rev. 123, 11488–11558 (2023).
Park, W. et al. In-depth correlation analysis between tear glucose and blood glucose using a wireless smart contact lens. Nat. Commun. 15, 2828 (2024). In this study, a wireless smart contact lens was successfully developed and validated for continuous, real-time monitoring of tear glucose, establishing a strong correlation with blood glucose through the concept of personalized lag time across multiple species and diabetic conditions.
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018). This article demonstrates a human pilot trial of ingestible electronic capsules capable of sensing oxygen, hydrogen and carbon dioxide in the gut, providing real-time data on gastrointestinal gas profiles and transit times.
Belknap, R. et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS ONE 8, e53373 (2013).
Siddiqui, I., Majid, H. & Abid, S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J. Gastrointest. Pharmacol. Ther. 8, 39–46 (2017).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
De la Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Mimee, M. et al. An ingestible bacterial–electronic system to monitor gastrointestinal health. Science 360, 915–918 (2018). This study reports on the development of an ingestible micro-bio-electronic device that combines engineered probiotic bacteria with miniaturized electronics for the in situ detection of gastrointestinal biomarkers, as validated in both in vitro and in vivo porcine models.
Inda-Webb, M. E. et al. Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ. Nature 620, 386–392 (2023).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Li, R. et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 11, 3207 (2020).
Ferguson, B. S. et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci. Transl. Med. 5, 213ra165 (2013).
Arroyo-Currás, N. et al. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl Acad. Sci. USA 114, 645–650 (2017).
Holmström, N., Nilsson, P., Carlsten, J. & Bowald, S. Long-term in vivo experience of an electrochemical sensor using the potential step technique for measurement of mixed venous oxygen pressure. Biosens. Bioelectron. 13, 1287–1295 (1998).
Theuns, D. A. M. J. et al. Prognostic role of high‐sensitivity C‐reactive protein and B‐type natriuretic peptide in implantable cardioverter‐defibrillator patients. Pacing Clin. Electrophysiol. 35, 275–282 (2012).
Abbassy, M. et al. Biosensors with left ventricular assist devices. Heart Fail. Rev. 29, 957–967 (2024).
Neubeck, L. et al. The mobile revolution—using smartphone apps to prevent cardiovascular disease. Nat. Rev. Cardiol. 12, 350–360 (2015).
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7, 168–179 (2024).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Song, Y. et al. 3D-printed epifluidic electronic skin for machine learning-powered multimodal health surveillance. Sci. Adv. 9, eadi6492 (2023).
Sundhoro, M. et al. Rapid and accurate electrochemical sensor for food allergen detection in complex foods. Sci. Rep. 11, 20831 (2021).
Criscuolo, F., Cantù, F., Taurino, I., Carrara, S. & De Micheli, G. A wearable electrochemical sensing system for non-invasive monitoring of lithium drug in bipolar disorder. IEEE Sens. J. 21, 9649–9656 (2021).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Alipour, A., Gabrielson, S. & Patel, P. B. Ingestible sensors and medication adherence: focus on use in serious mental illness. Pharmacy 8, 103 (2020).
Kane, J. M. et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J. Clin. Psychiatry 74, e533–e540 (2013).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Xu, C., Solomon, S. A. & Gao, W. Artificial intelligence-powered electronic skin. Nat. Mach. Intell. 5, 1344–1355 (2023).
Flynn, C. D. & Chang, D. Artificial intelligence in point-of-care biosensing: challenges and opportunities. Diagnostics 14, 1100 (2024).
Rodbard, D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol. Ther. 19, S25–S37 (2017).
Soto, R. J., Hall, J. R., Brown, M. D., Taylor, J. B. & Schoenfisch, M. H. In vivo chemical sensors: role of biocompatibility on performance and utility. Anal. Chem. 89, 276–299 (2016).
Chang, D. et al. A high-dimensional microfluidic approach for selection of aptamers with programmable binding affinities. Nat. Chem. 15, 773–780 (2023).
Zhu, Y. et al. Lab-on-a-contact lens: recent advances and future opportunities in diagnostics and therapeutics. Adv. Mat. 34, 2108389 (2022).
Smith, J. L. & Rice, M. J. Why have so many intravascular glucose monitoring devices failed? J. Diabetes Sci. Technol. 9, 782–791 (2015).
Sideri, K. et al. Digital pills for the remote monitoring of medication intake: a stakeholder analysis and assessment of marketing approval and patent granting policies. J. Law Biosci. 9, lsac029 (2022).
De Miguel Beriain, I. & Morla González, M. ‘Digital pills’ for mental diseases: an ethical and social analysis of the issues behind the concept. J. Law Biosci. 7, lsaa040 (2020).
Tadikonda, S. HealthVerity: Real World Data and Evidence. Case 824-019 (Harvard Business School, 2023).
Galindo, R. J. et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J. Diabetes Sci. Technol. 14, 1035–1064 (2020).
Tu, J. & Gao, W. Ethical considerations of wearable technologies in human research. Adv. Healthc. Mater. 10, 2100127 (2021).
Zheng, H. et al. Reverse iontophoresis with the development of flexible electronics: a review. Biosens. Bioelectron. 223, 115036 (2023).
Wang, C. et al. A microfluidic wearable device for wound exudate management and analysis in human chronic wounds. Sci. Transl. Med. 17, eadt0882 (2025).
Shibasaki, K., Kimura, M., Ikarashi, R., Yamaguchi, A. & Watanabe, T. Uric acid concentration in saliva and its changes with the patients receiving treatment for hyperuricemia. Metabolomics 8, 484–491 (2012).
Daum, K. M. & Hill, R. M. Human tear glucose. Invest. Ophthalmol. Vis. Sci. 22, 509–514 (1982).
Min, J. et al. Continuous biochemical profiling of the gastrointestinal tract using an integrated smart capsule. Nat. Electron. https://doi.org/10.1038/s41928-025-01407-0 (2025).
Barton, M. & Yanagisawa, M. Endothelin: 30 years from discovery to therapy. Hypertension 74, 1232–1265 (2019).
Tektonidou, M. G. & Ward, M. M. Validation of new biomarkers in systemic autoimmune diseases. Nat. Rev. Rheumatol. 7, 708–717 (2011).
PRECISION study: evaluating the accuracy of the LabPatch continuous glucose monitor. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03262415 (2023).
A study of non-invasive measurement of blood glucose and blood pressure. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05904769?intr=LIFELEAF&rank=1 (2025).
Epicore Biosystems launches Discovery Patch® Sweat Collection System. Epicore Biosystems https://www.prnewswire.com/news-releases/epicore-biosystems-launches-discovery-patch-sweat-collection-system-301392407.html (2021).
Law, R. Biolinq granted de novo classification for needle-free glucose monitor. Medical Device Network https://www.medicaldevice-network.com/news/biolinq-granted-de-novo-classification-for-needle-free-glucose-monitor (2025).
Eversense E3 Continuous Glucose Monitoring (CGM) System – P160048/S021 FDA https://www.fda.gov/medical-devices/recently-approved-devices/eversense-e3-continuous-glucose-monitoring-cgm-system-p160048s021 (2023).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Das, J. et al. Reagentless biomolecular analysis using a molecular pendulum. Nat. Chem. 13, 428–434 (2021). This article introduces a novel molecular pendulum sensing mechanism capable of the reagentless detection of diverse protein biomarkers in multiple biofluids, enabling continuous real-time monitoring and in vivo measurements.
Zargartalebi, H. et al. Capillary-assisted molecular pendulum bioanalysis. J. Am. Chem. Soc. 144, 18338–18349 (2022).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Lu, Z. et al. Biomolecule sensors based on organic electrochemical transistors. NPJ Flex. Electron. 9, 9 (2025).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Lee, K. H. et al. Synergistic SERS enhancement in GaN–Ag hybrid system toward label-free and multiplexed detection of antibiotics in aqueous solutions. Adv. Sci. 8, 2100640 (2021).
Xu, K., Zhou, R., Takei, K. & Hong, M. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. 6, 1900925 (2019).
Wang, Y. et al. Wearable plasmonic-metasurface sensor for noninvasive and universal molecular fingerprint detection on biointerfaces. Sci. Adv. 7, eabe4553 (2021).
Lin, L. & Wang, L. V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022).
Zargartalebi, H. et al. Active-reset protein sensors enable continuous in vivo monitoring of inflammation. Science 386, 1146–1153 (2024). This article introduces an active-reset methodology that enables receptor regeneration through the application of an alternating electric potential and facilitates continuous protein monitoring.
Sun, N. et al. Aptamer melting biosensors for thousands of signaling and regenerating cycles. Biosens. Bioelectron. 271, 116998 (2025).
Acknowledgements
This project was supported by the National Science Foundation (grants 2145802 and 2444815), National Institutes of Health (grants R01HL155815, R01HL165002 and R21DK13266), Army Research Office (grant W911NF-23-1-0041), American Cancer Society Research Scholar (grant RSG-21-181-01-CTPS), US Army Medical Research Acquisition Activity (grant HT9425-24-1-0249), Advanced Research Projects Agency for Health Sprint for Women’s Health (award number ARPA-H-ICHUB-24-101-504), Heritage Medical Research Institute, Natural Sciences and Engineering Research Council of Canada and Chan Zuckerberg Biohub Chicago.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, J., Flynn, C.D., Yeom, J. et al. Wearable biomolecular sensing nanotechnologies in chronic disease management. Nat. Nanotechnol. 20, 1388–1404 (2025). https://doi.org/10.1038/s41565-025-02010-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-02010-2