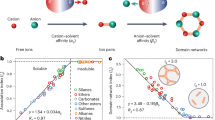
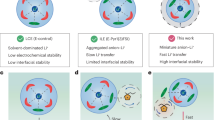
Abstract
Research and development of non-aqueous electrolyte solutions are essential for practical advancement towards the production of high-energy lithium metal batteries (LMBs). An ideal LMB electrolyte solution should enable highly efficient, uniform and prolonged lithium metal plating and stripping, preserve the electrodes’ electro(chemo)mechanical properties and ensure compatibility with all cell components. However, despite extensive research efforts, scientists have yet to achieve an electrolyte design that meets these requirements simultaneously. Here, by examining the nanoengineering aspects of various non-aqueous electrolyte solution designs, we elucidate the understanding of the nanoscale physicochemical and electrochemical processes taking place in LMBs, which are mainly governed by the thermodynamic and kinetic properties of the electrolyte system. We also explore emerging research directions and propose an accelerated, iterative framework that integrates nanoengineering principles with machine learning, high-throughput computation and experimentation to facilitate the development of next-generation non-aqueous electrolyte solutions for practical LMBs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Horstmann, B. et al. Strategies towards enabling lithium metal in batteries: interphases and electrodes. Energy Environ. Sci. 14, 5289–5314 (2021).
Brandt, K. & Laman, F. C. Reproducibility and reliability of rechargeable lithium/molybdenum disulfide batteries. J. Power Sources 25, 265–276 (1989).
Fang, C., Wang, X. & Meng, Y. S. Key issues hindering a practical lithium-metal anode. Trends Chem. 1, 152–158 (2019).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Jagger, B. & Pasta, M. Solid electrolyte interphases in lithium metal batteries. Joule 7, 2228–2244 (2023).
He, X. et al. The passivity of lithium electrodes in liquid electrolytes for secondary batteries. Nat. Rev. Mater. 6, 1036–1052 (2021).
Lu, D. et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Wang, H. et al. Liquid electrolyte: the nexus of practical lithium metal batteries. Joule 6, 588–616 (2022).
Boyle, D. T. et al. Correlating kinetics to cyclability reveals thermodynamic origin of lithium anode morphology in liquid electrolytes. J. Am. Chem. Soc. 144, 20717–20725 (2022).
Giffin, G. A. The role of concentration in electrolyte solutions for non-aqueous lithium-based batteries. Nat. Commun. 13, 5250 (2022).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022). Systematic design of bi-ethers to optimize the thermodynamic and kinetic properties of liquid electrolytes.
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Choi, I. R. et al. Asymmetric ether solvents for high-rate lithium metal batteries. Nat. Energy 10, 365–379 (2025).
Zhang, G. et al. A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries. Nat. Commun. 14, 1081 (2023). Single-solvent mono-ether-based electrolyte enabling efficient Li stripping/plating at high current densities.
Yang, W., Chen, A., He, P. & Zhou, H. Advancing lithium metal electrode beyond 99.9% coulombic efficiency via super-saturated electrolyte with compressed solvation structure. Nat. Commun. 16, 4229 (2025).
Xu, K. Electrolytes, Interfaces and Interphases (Royal Society of Chemistry, 2023).
Zhou, P., Xiang, Y. & Liu, K. Understanding and applying the donor number of electrolytes in lithium metal batteries. Energy Environ. Sci. 17, 8057–8077 (2024).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Peljo, P. & Girault, H. H. Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception. Energy Environ. Mater. 11, 2306–2309 (2018).
Xu, K., Ding, S. P. & Jow, T. R. Toward reliable values of electrochemical stability limits for electrolytes. J. Electrochem. Soc. 146, 4172–4178 (1999).
Sethurajan, A. K., Krachkovskiy, S. A., Halalay, I. C., Goward, G. R. & Protas, B. Accurate characterization of ion transport properties in binary symmetric electrolytes using in situ NMR imaging and inverse modeling. J. Phys. Chem. B 119, 12238–12248 (2015).
Hou, T. & Monroe, C. W. Composition-dependent thermodynamic and mass-transport characterization of lithium hexafluorophosphate in propylene carbonate. Electrochim. Acta 332, 135085 (2020).
Wang, A. A., Hou, T., Karanjavala, M. & Monroe, C. W. Shifting-reference concentration cells to refine composition-dependent transport characterization of binary lithium-ion electrolytes. Electrochim. Acta 358, 136688 (2020).
Diederichsen, K. M., McShane, E. J. & McCloskey, B. D. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2, 2563–2575 (2017).
Lorenz, M. et al. Local volume conservation in concentrated electrolytes is governing charge transport in electric fields. J. Phys. Chem. Lett. 13, 8761–8767 (2022).
Schammer, M., Horstmann, B. & Latz, A. Theory of transport in highly concentrated electrolytes. J. Electrochem. Soc. 168, 026511 (2021).
Zugmann, S. et al. Measurement of transference numbers for lithium ion electrolytes via four different methods, a comparative study. Electrochim. Acta 56, 3926–3933 (2011).
Petrowsky, M., Frech, R., Suarez, S. N., Jayakody, J. R. P. & Greenbaum, S. Investigation of fundamental transport properties and thermodynamics in diglyme−salt solutions. J. Phys. Chem. B 110, 23012–23021 (2006).
Kwabi, D. G. et al. Experimental and computational analysis of the solvent-dependent O2/Li+–O2− redox couple: standard potentials, coupling strength, and implications for lithium–oxygen batteries. Angew. Chem. Int. Ed. 55, 3129–3134 (2016).
Leverick, G. & Shao-Horn, Y. Controlling electrolyte properties and redox reactions using solvation and implications in battery functions: a mini-review. Adv. Energy Mater. 13, 2204094 (2023).
Ko, S. et al. Electrode potential influences the reversibility of lithium-metal anodes. Nat. Energy 7, 1217–1224 (2022).
Wu, Q., McDowell, M. T. & Qi, Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries. J. Am. Chem. Soc. 145, 2473–2484 (2023).
Angarita-Gomez, S. & Balbuena, P. B. Solvation vs. surface charge transfer: an interfacial chemistry game drives cation motion. Chem. Commun. 57, 6189–6192 (2021).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Camacho-Forero, L. E., Smith, T. W. & Balbuena, P. B. Effects of high and low salt concentration in electrolytes at lithium-metal anode surfaces. J. Phys. Chem. C 121, 182–194 (2017).
Sayah, S. et al. How do super concentrated electrolytes push the Li-ion batteries and supercapacitors beyond their thermodynamic and electrochemical limits?. Nano Energy 98, 107336 (2022).
Dokko, K. et al. Direct evidence for Li ion hopping conduction in highly concentrated sulfolane-based liquid electrolytes. J. Phys. Chem. B 122, 10736–10745 (2018).
Raccichini, R., Dibden, J. W., Brew, A., Owen, J. R. & García-Aráez, N. Ion speciation and transport properties of LiTFSI in 1,3-dioxolane solutions: a case study for Li–S battery applications. J. Phys. Chem. B 122, 267–274 (2018).
Chen, Y. et al. Steric effect tuned ion solvation enabling stable cycling of high-voltage lithium metal battery. J. Am. Chem. Soc. 143, 18703–18713 (2021).
Lin, Y.-X. et al. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 309, 221–230 (2016).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Wang, M. et al. Effect of LiFSI concentrations to form thickness- and modulus-controlled SEI layers on lithium metal anodes. J. Phys. Chem. C 122, 9825–9834 (2018).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Li, Y. & Qi, Y. Transferable self-consistent charge density functional tight-binding parameters for Li-metal and Li-ions in inorganic compounds and organic solvents. J. Phys. Chem. C 122, 10755–10764 (2018).
Soto, F. A., Ma, Y., Martinez De La Hoz, J. M., Seminario, J. M. & Balbuena, P. B. Formation and growth mechanisms of solid–electrolyte interphase layers in rechargeable batteries. Chem. Mater. 27, 7990–8000 (2015).
Single, F., Latz, A. & Horstmann, B. Identifying the mechanism of continued growth of the solid-electrolyte interphase. ChemSusChem 11, 1950–1955 (2018).
Von Kolzenberg, L., Latz, A. & Horstmann, B. Solid–electrolyte interphase during battery cycling: theory of growth regimes. ChemSusChem 13, 3901–3910 (2020).
Single, F., Horstmann, B. & Latz, A. Dynamics and morphology of solid electrolyte interphase (SEI). Phys. Chem. Chem. Phys. 18, 17810–17814 (2016).
Single, F., Horstmann, B. & Latz, A. Revealing SEI morphology: in-depth analysis of a modeling approach. J. Electrochem. Soc. 164, E3132–E3145 (2017).
Harris, O. C., Lin, Y., Qi, Y., Leung, K. & Tang, M. H. How transition metals enable electron transfer through the SEI: part I. Experiments and Butler–Volmer modeling. J. Electrochem. Soc. 167, 013502 (2020).
Menkin, S. et al. Toward an understanding of SEI formation and lithium plating on copper in anode-free batteries. J. Phys. Chem. C 125, 16719–16732 (2021).
Wang, H. et al. The effect of removing the native passivation film on the electrochemical performance of lithium metal electrodes. J. Power Sources 520, 230817 (2022).
Kühn, S. P. et al. Back to the basics: advanced understanding of the as-defined solid electrolyte interphase on lithium metal electrodes. J. Power Sources 549, 232118 (2022).
Otto, S.-K. et al. Storage of lithium metal: the role of the native passivation layer for the anode interface resistance in solid state batteries. ACS Appl. Energy Mater. 4, 12798–12807 (2021).
Yoon, J. S. et al. Thermodynamics, adhesion, and wetting at Li/Cu(-oxide) interfaces: relevance for anode-free lithium-metal batteries. ACS Appl. Mater. Interfaces 16, 18790–18799 (2024).
Aravindan, V., Gnanaraj, J., Madhavi, S. & Liu, H. Lithium-ion conducting electrolyte salts for lithium batteries. Chem. Eur. J. 17, 14326–14346 (2011).
Schmitz, R. W. et al. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 42, 65–84 (2014).
Yeddala, M., Rynearson, L. & Lucht, B. L. Modification of carbonate electrolytes for lithium metal electrodes. ACS Energy Lett. 8, 4782–4793 (2023).
Bai, P., Li, J., Brushett, F. R. & Bazant, M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016).
Shin, W. & Manthiram, A. A facile potential hold method for fostering an inorganic solid-electrolyte interphase for anode-free lithium-metal batteries. Angew. Chem. 134, e202115909 (2022).
Kwon, Y. et al. Elucidating the role of cathode identity: voltage-dependent reversibility of anode-free batteries. Chem 10, 3159–3183 (2024).
Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021).
Lei, Y. et al. Recent advances in separator design for lithium metal batteries without dendrite formation: implications for electric vehicles. eTransportation 20, 100330 (2024).
Ishikawa, M., Tasaka, Y., Yoshimoto, N. & Morita, M. Optimization of physicochemical characteristics of a lithium anode interface for high-efficiency cycling: an effect of electrolyte temperature. J. Power Sources 97/98, 262–264 (2001).
Wang, J. et al. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019).
Sheng, S., Sheng, L., Wang, L., Piao, N. & He, X. Thickness variation of lithium metal anode with cycling. J. Power Sources 476, 228749 (2020).
McBrayer, J. D., Apblett, C. A., Harrison, K. L., Fenton, K. R. & Minteer, S. D. Mechanical studies of the solid electrolyte interphase on anodes in lithium and lithium ion batteries. Nanotechnology 32, 502005 (2021).
Yuan, S. et al. Revisiting the designing criteria of advanced solid electrolyte interphase on lithium metal anode under practical condition. Nano Energy 83, 105847 (2021).
Shen, X. et al. The failure of solid electrolyte interphase on Li metal anode: structural uniformity or mechanical strength? Adv. Energy Mater. 10, 1903645 (2020).
Werres, M. et al. Origin of heterogeneous stripping of lithium in liquid electrolytes. ACS Nano 17, 10218–10228 (2023).
Gao, Y. et al. Unraveling the mechanical origin of stable solid electrolyte interphase. Joule 5, 1860–1872 (2021).
Gu, Y. et al. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 9, 1339 (2018).
Wang, J. et al. In situ self-assembly of ordered organic/inorganic dual-layered interphase for achieving long-life dendrite-free Li metal anodes in LiFSI-based electrolyte. Adv. Funct. Mater. 31, 2007434 (2021).
Xu, Y. et al. Theoretical calculation study on the electrochemical properties of lithium halide-based artificial SEI films for lithium metal anodes. Surf. Interfaces 44, 103768 (2024).
Shi, S. et al. Direct calculation of Li-ion transport in the solid electrolyte interphase. J. Am. Chem. Soc. 134, 15476–15487 (2012).
Lu, P. & Harris, S. J. Lithium transport within the solid electrolyte interphase. Electrochem. Commun. 13, 1035–1037 (2011). Investigation of Li+ transport in the SEI via isotope exchange experiments.
Yu, X. et al. Direct and in situ examination of Li+ transport kinetics in an isotope-labeled solid–electrolyte interphase. Proc. Natl Acad. Sci. USA 122, e2514652122 (2025).
Das Goswami, B. R., Jabbari, V., Shahbazian-Yassar, R., Mashayek, F. & Yurkiv, V. Unraveling ion diffusion pathways and energetics in polycrystalline SEI of lithium-based batteries: combined cryo-HRTEM and DFT study. J. Phys. Chem. C 127, 21971–21979 (2023).
Soto, F. A., Marzouk, A., El-Mellouhi, F. & Balbuena, P. B. Understanding ionic diffusion through SEI components for lithium-ion and sodium-ion batteries: insights from first-principles calculations. Chem. Mater. 30, 3315–3322 (2018).
Xu, Y. et al. Direct in situ measurements of electrical properties of solid-electrolyte interphase on lithium metal anodes. Nat. Energy 8, 1345–1354 (2023). Experimental evidence of the electrical semiconducting properties of the SEI.
Benitez, L. & Seminario, J. M. Electron transport and electrolyte reduction in the solid-electrolyte interphase of rechargeable lithium ion batteries with silicon anodes. J. Phys. Chem. C 120, 17978–17988 (2016).
Derosa, P. A. & Seminario, J. M. Electron transport through single molecules: scattering treatment using density functional and Green function theories. J. Phys. Chem. B 105, 471–481 (2001).
Köbbing, L., Latz, A. & Horstmann, B. Growth of the solid-electrolyte interphase: electron diffusion versus solvent diffusion. J. Power Sources 561, 232651 (2023).
Feng, M., Pan, J. & Qi, Y. Impact of electronic properties of grain boundaries on the solid electrolyte interphases (SEIs) in Li-ion batteries. J. Phys. Chem. C 125, 15821–15829 (2021).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Steiger, J., Kramer, D. & Mönig, R. Mechanisms of dendritic growth investigated by in situ light microscopy during electrodeposition and dissolution of lithium. J. Power Sources 261, 112–119 (2014).
Xu, Y. et al. Current density regulated atomic to nanoscale process on Li deposition and solid electrolyte interphase revealed by cryogenic transmission electron microscopy. ACS Nano 14, 8766–8775 (2020).
Boyle, D. T. et al. Resolving current-dependent regimes of electroplating mechanisms for fast charging lithium metal anodes. Nano Lett. 22, 8224–8232 (2022).
He, M., Guo, R., Hobold, G. M., Gao, H. & Gallant, B. M. The intrinsic behavior of lithium fluoride in solid electrolyte interphases on lithium. Proc. Natl Acad. Sci. USA 117, 73–79 (2020).
Zhang, X.-Q., Cheng, X.-B., Chen, X., Yan, C. & Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 27, 1605989 (2017).
Dhattarwal, H. S., Kuo, J.-L. & Kashyap, H. K. Mechanistic insight on the stability of ether and fluorinated ether solvent-based lithium bis(fluoromethanesulfonyl) electrolytes near Li metal surface. J. Phys. Chem. C 126, 8953–8963 (2022).
Perez-Beltran, S., Kuai, D. & Balbuena, P. B. SEI formation and lithium-ion electrodeposition dynamics in lithium metal batteries via first-principles kinetic Monte Carlo modeling. ACS Energy Lett. 9, 5268–5278 (2024).
Tan, Y. et al. Lithium fluoride in electrolyte for stable and safe lithium-metal batteries. Adv. Mater. 33, 2102134 (2021).
Zeng, H. et al. Beyond LiF: tailoring Li2O-dominated solid electrolyte interphase for stable lithium metal batteries. ACS Nano 18, 1969–1981 (2024).
Hobold, G. M., Wang, C., Steinberg, K., Li, Y. & Gallant, B. M. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high Coulombic efficiency. Nat. Energy 9, 580–591 (2024). Correlation of Li2O prevalence in the SEI and the CE in lithium metal batteries.
Gao, K., Sun, L., Wang, K. & Zhang, Y. Non-aqueous liquid electrolytes in lithium metal battery: components and modification. Mater. Today Energy 37, 101413 (2023).
Borodin, O., Self, J., Persson, K. A., Wang, C. & Xu, K. Uncharted waters: super-concentrated electrolytes. Joule 4, 69–100 (2020).
Jiang, G. et al. Perspective on high-concentration electrolytes for lithium metal batteries. Small Struct. 2, 2000122 (2021).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Ren, X. et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018). Introduction of LHCEs as promising electrolyte concept for lithium metal batteries.
Zheng, J. et al. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 3, 315–321 (2018).
Efaw, C. M. et al. Localized high-concentration electrolytes get more localized through micelle-like structures. Nat. Mater. 22, 1531–1539 (2023).
Verma, A., Schulze, M. C. & Colclasure, A. Micelle-like bulk structure of localized high-concentration electrolytes. Joule 8, 10–12 (2024).
Cao, X., Jia, H., Xu, W. & Zhang, J.-G. Review—Localized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 168, 010522 (2021).
Chen, J. et al. Design of localized high-concentration electrolytes via donor number. ACS Energy Lett. 8, 1723–1734 (2023).
Ren, F. et al. Solvent–diluent interaction-mediated solvation structure of localized high-concentration electrolytes. ACS Appl. Mater. Interfaces 14, 4211–4219 (2022).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Zhang, X. et al. Advanced electrolytes for fast-charging high-voltage lithium-ion batteries in wide-temperature range. Adv. Energy Mater. 10, 2000368 (2020).
Jia, H. et al. High-performance silicon anodes enabled by nonflammable localized high-concentration electrolytes. Adv. Energy Mater. 9, 1900784 (2019).
Ahmed, R. A. et al. Enhanced electrochemical performance of disordered rocksalt cathodes in a localized high-concentration electrolyte. Adv. Energy Mater. 14, 2400722 (2024).
Cao, X. et al. Optimization of fluorinated orthoformate based electrolytes for practical high-voltage lithium metal batteries. Energy Storage Mater. 34, 76–84 (2021).
Cao, X. Effects of fluorinated solvents on electrolyte solvation structures and electrode/electrolyte interphases for lithium metal batteries. Proc. Natl Acad. Sci. USA 118, e2020357118 (2021).
Niu, C. et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 6, 723–732 (2021).
Perez Beltran, S., Cao, X., Zhang, J.-G., El-Khoury, P. Z. & Balbuena, P. B. Influence of diluent concentration in localized high concentration electrolytes: elucidation of hidden diluent–Li + interactions and Li + transport mechanism. J. Mater. Chem. A 9, 17459–17473 (2021).
Liu, Y. et al. Regulating electrolyte solvation structures via diluent–solvent interactions for safe high-voltage lithium metal batteries. Small 20, 2311812 (2024).
Zhao, Y. et al. Electrolyte engineering for highly inorganic solid electrolyte interphase in high-performance lithium metal batteries. Chem 9, 682–697 (2023).
Shi, J. et al. An amphiphilic molecule-regulated core–shell-solvation electrolyte for Li-metal batteries at ultra-low temperature. Angew. Chem. Int. Ed. 62, e202218151 (2023).
Kim, S. et al. Wide-temperature-range operation of lithium-metal batteries using partially and weakly solvating liquid electrolytes. Energy Environ. Sci. 16, 5108–5122 (2023).
Tran, T. et al. Enhancing cycling stability of lithium metal batteries by a bifunctional fluorinated ether. Adv. Funct. Mater. 34, 2407012 (2024).
Chen, S. et al. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2, 1548–1558 (2018).
Cao, N. et al. Designing ionic liquid electrolytes for a rigid and Li+-conductive solid electrolyte interface in high performance lithium metal batteries. Chem. Phys. Lett. 866, 141959 (2025).
Hai, F. et al. A low-cost, fluorine-free localized highly concentrated electrolyte toward ultra-high loading lithium metal batteries. Adv. Energy Mater. 14, 2304253 (2024).
Yuan, Z., Chen, A., Liao, J., Song, L. & Zhou, X. Recent advances in multifunctional generalized local high-concentration electrolytes for high-efficiency alkali metal batteries. Nano Energy 119, 109088 (2024).
Li, M. et al. Acetonitrile-based local high-concentration electrolytes for advanced lithium metal batteries. Adv. Mater. 36, 2404271 (2024).
Jie, Y. et al. Towards long-life 500 Wh kg−1 lithium metal pouch cells via compact ion-pair aggregate electrolytes. Nat. Energy 9, 987–998 (2024).
Kim, S. C. et al. High-entropy electrolytes for practical lithium metal batteries. Nat. Energy 8, 814–826 (2023).
Li, Z. et al. Critical review of fluorinated electrolytes for high-performance lithium metal batteries. Adv. Funct. Mater. 33, 2300502 (2023).
Wichmann, L. et al. Design of fluorine-free weakly coordinating electrolyte solvents with enhanced oxidative stability. Angew. Chem. Int. Ed. 64, e202506826 (2025).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Zhang, X. et al. Li+(ionophore) nanoclusters engineered aqueous/non-aqueous biphasic electrolyte solutions for high-potential lithium-based batteries. Nat. Nanotechnol. 20, 798–806 (2025).
Vu, M. C. et al. Low melting alkali-based molten salt electrolytes for solvent-free lithium-metal batteries. Matter 6, 4357–4375 (2023). Report of low melting FSI-based molten salt electrolyte with high oxidative stability, enabling high Coulombic efficiencies at high rates.
Xue, W. et al. FSI-inspired solvent and ‘full fluorosulfonyl’ electrolyte for 4 V class lithium-metal batteries. Energy Environ. Sci. 13, 212–220 (2020). Introduction of full fluorosulfonyl electrolytes for lithium metal batteries.
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Rustomji, C. S. et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017). Report of liquefied gas electrolytes enabling efficient Li plating/stripping.
Yang, Y. et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019).
Louli, A. J. et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020).
Weber, R. et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019).
Qiu, F. et al. A concentrated ternary-salts electrolyte for high reversible Li metal battery with slight excess Li. Adv. Energy Mater. 9, 1803372 (2019).
Kang, D. W., Moon, J., Choi, H.-Y., Shin, H.-C. & Kim, B. G. Stable cycling and uniform lithium deposition in anode-free lithium-metal batteries enabled by a high-concentration dual-salt electrolyte with high LiNO3 content. J. Power Sources 490, 229504 (2021).
Stuckenberg, S. et al. Influence of LiNO3 on the lithium metal deposition behavior in carbonate-based liquid electrolytes and on the electrochemical performance in zero-excess lithium metal batteries. Small 20, 2305203 (2024).
Agostini, M., Scrosati, B. & Hassoun, J. An advanced lithium-ion sulfur battery for high energy storage. Adv. Energy Mater. 5, 1500481 (2015).
Ma, Q. et al. Improved cycling stability of lithium-metal anode with concentrated electrolytes based on lithium (fluorosulfonyl)(trifluoromethanesulfonyl)imide. ChemElectroChem 3, 531–536 (2016).
Weintz, D., Kühn, S. P., Winter, M. & Cekic-Laskovic, I. Tailoring the preformed solid electrolyte interphase in lithium metal batteries: impact of fluoroethylene carbonate. ACS Appl. Mater. Interfaces 15, 53526–53532 (2023).
Xue, T. et al. Tailoring fluorine-rich solid electrolyte interphase to boost high efficiency and long cycling stability of lithium metal batteries. Sci. China Chem. 66, 2121–2129 (2023).
Ding, F. et al. Effects of cesium cations in lithium deposition via self-healing electrostatic shield mechanism. J. Phys. Chem. C 118, 4043–4049 (2014).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Frith, J. T., Lacey, M. J. & Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 14, 420 (2023).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Single, F., Horstmann, B. & Latz, A. Theory of impedance spectroscopy for lithium batteries. J. Phys. Chem. C 123, 27327–27343 (2019).
Stolz, L., Winter, M. & Kasnatscheew, J. Practical relevance of charge transfer resistance at the Li metal electrode|electrolyte interface in batteries?. J. Solid State Electrochem. 29, 4181–4186 (2025).
Meddings, N. et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: a review. J. Power Sources 480, 228742 (2020).
Meunier, V., Leal De Souza, M., Morcrette, M. & Grimaud, A. Design of workflows for crosstalk detection and lifetime deviation onset in Li-ion batteries. Joule 7, 42–56 (2023).
Meng, W. et al. The progress of in situ technology for lithium metal batteries. Mater. Chem. Front. 8, 700–714 (2024).
Scurtu, R.-G. et al. From small batteries to big claims. Nat. Nanotechnol. 20, 970–976 (2025).
Xu, Y. et al. Atomic to nanoscale origin of vinylene carbonate enhanced cycling stability of lithium metal anode revealed by cryo-transmission electron microscopy. Nano Lett. 20, 418–425 (2020).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Chen, W. et al. Formation and impact of nanoscopic oriented phase domains in electrochemical crystalline electrodes. Nat. Mater. 22, 92–99 (2023).
Ji, P., Lei, X. & Su, D. In situ transmission electron microscopy methods for lithium-ion batteries. Small Methods 8, 2301539 (2024).
Zhang, Z. et al. Characterizing batteries by in situ electrochemical atomic force microscopy: a critical review. Adv. Energy Mater. 11, 2101518 (2021).
Wolff, B. & Hausen, F. Mechanical evolution of solid electrolyte interphase on metallic lithium studied by in situ atomic force microscopy. J. Electrochem. Soc. 170, 010534 (2023).
Tan, S. et al. Evolution and interplay of lithium metal interphase components revealed by experimental and theoretical studies. J. Am. Chem. Soc. 146, 11711–11718 (2024).
Ma, C., Xu, F. & Song, T. Dual-layered interfacial evolution of lithium metal anode: SEI analysis via TOF-SIMS technology. ACS Appl. Mater. Interfaces 14, 20197–20207 (2022).
Markevich, E., Salitra, G., Chesneau, F., Schmidt, M. & Aurbach, D. Very stable lithium metal stripping–plating at a high rate and high areal capacity in fluoroethylene carbonate-based organic electrolyte solution. ACS Energy Lett. 2, 1321–1326 (2017).
Schmitz, R. et al. SEI investigations on copper electrodes after lithium plating with Raman spectroscopy and mass spectrometry. J. Power Sources 233, 110–114 (2013).
Hope, M. A. et al. Selective NMR observation of the SEI–metal interface by dynamic nuclear polarisation from lithium metal. Nat. Commun. 11, 2224 (2020).
Hsieh, Y.-C. et al. Quantification of dead lithium via in situ nuclear magnetic resonance spectroscopy. Cell Rep. Phys. Sci. 1, 100139 (2020).
Golozar, M. et al. In situ observation of solid electrolyte interphase evolution in a lithium metal battery. Commun. Chem. 2, 131 (2019).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
He, X., Larson, J. M., Bechtel, H. A. & Kostecki, R. In situ infrared nanospectroscopy of the local processes at the Li/polymer electrolyte interface. Nat. Commun. 13, 1398 (2022).
Zhang, H., Shen, C., Huang, Y. & Liu, Z. Spontaneously formation of SEI layers on lithium metal from LiFSI/DME and LiTFSI/DME electrolytes. Appl. Surf. Sci. 537, 147983 (2021).
Perez Beltran, S. & Balbuena, P. B. SEI formation mechanisms and Li+ dissolution in lithium metal anodes: impact of the electrolyte composition and the electrolyte-to-anode ratio. J. Power Sources 551, 232203 (2022).
Wagner-Henke, J. et al. Knowledge-driven design of solid-electrolyte interphases on lithium metal via multiscale modelling. Nat. Commun. 14, 6823 (2023).
Pohlmann, S. Metrics and methods for moving from research to innovation in energy storage. Nat. Commun. 13, 1538 (2022).
Benayad, A. et al. High-throughput experimentation and computational freeway lanes for accelerated battery electrolyte and interface development research. Adv. Energy Mater. 12, 2102678 (2022).
Ward, L. et al. Principles of the Battery Data Genome. Joule 6, 2253–2271 (2022).
Qu, X. et al. The Electrolyte Genome project: a big data approach in battery materials discovery. Comput. Mater. Sci. 103, 56–67 (2015).
Tagade, P. M. et al. Attribute driven inverse materials design using deep learning Bayesian framework. npj Comput. Mater. 5, 127 (2019).
Barter, D. et al. Predictive stochastic analysis of massive filter-based electrochemical reaction networks. Digit. Discov. 2, 123–137 (2023).
Gao, Y.-C. et al. Data-driven insight into the reductive stability of ion–solvent complexes in lithium battery electrolytes. J. Am. Chem. Soc. 145, 23764–23770 (2023).
Yan, P. et al. Non-aqueous battery electrolytes: high-throughput experimentation and machine learning-aided optimization of ionic conductivity. J. Mater. Chem. A 12, 19123–19136 (2024).
Dave, A. et al. Autonomous optimization of non-aqueous Li-ion battery electrolytes via robotic experimentation and machine learning coupling. Nat. Commun. 13, 5454 (2022).
Flores, E. et al. Learning the laws of lithium-ion transport in electrolytes using symbolic regression. Digit. Discov. 1, 440–447 (2022).
Lewis, G. N. & Keyes, F. G. The potential of the lithium electrode. J. Am. Chem. Soc. 35, 340–344 (1913).
Harris, W. S. Electrochemical Studies in Cyclic Esters. PhD thesis, Univ. California, Berkeley (1958). Demonstration of reversible electrochemical Li deposition and dissolution.
Greatbatch, W. et al. The solid-state lithium battery: a new improved chemical power source for implantable cardiac pacemakers. IEEE Trans. Biomed. Eng BME-18, 317–324 (1971).
Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J. Electrochem. Soc. 126, 2047–2051 (1979). Proposal of the SEI model.
Scarr, R. F. Kinetics of the solid lithium electrode in propylene carbonate. J. Electrochem. Soc. 117, 295–298 (1970).
Winter, M., Barnett, B. & Xu, K. Before Li ion batteries. Chem. Rev. 118, 11433–11456 (2018).
Selim, R. & Bro, P. Some observations on rechargeable lithium electrodes in a propylene carbonate electrolyte. J. Electrochem. Soc. 121, 1457–1459 (1974).
Rauh, R. D. & Brummer, S. B. The effect of additives on lithium cycling in propylene carbonate. Electrochim. Acta 22, 75–83 (1977).
Koch, V. R. & Young, J. H. The stability of the secondary lithium electrode in tetrahydrofuran-based electrolytes. J. Electrochem. Soc. 125, 1371–1377 (1978).
Koch, V. R. & Young, J. H. 2-Methyltetrahydrofuran–lithium hexafluoroarsenate: a superior electrolyte for the secondary lithium electrode. Science 204, 499–501 (1979).
Koch, V. R., Goldman, J. L., Mattos, C. J. & Mulvaney, M. Specular lithium deposits from lithium hexafluoroarsenate/diethyl ether electrolytes. J. Electrochem. Soc. 129, 1–4 (1982).
Ding, F. et al. Effects of carbonate solvents and lithium salts on morphology and Coulombic efficiency of lithium electrode. J. Electrochem. Soc. 160, A1894–A1901 (2013).
Miao, R. et al. Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility. J. Power Sources 271, 291–297 (2014).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Zhao, Y., Zhou, T., Mensi, M., Choi, J. W. & Coskun, A. Electrolyte engineering via ether solvent fluorination for developing stable non-aqueous lithium metal batteries. Nat. Commun. 14, 299 (2023).
Li, C. et al. Developing diluted low-concentration electrolyte with a high anion-to-solvent ratio for high-voltage lithium metal batteries. J. Mater. Chem. A 12, 8236–8243 (2024).
Morita, M., Asai, Y., Yoshimoto, N. & Ishikawa, M. A Raman spectroscopic study of organic electrolyte solutions based on binary solvent systems of ethylene carbonate with low viscosity solvents which dissolve different lithium salts. J. Chem. Soc. Faraday Trans. 94, 3451–3456 (1998).
Qian, K., Winans, R. E. & Li, T. Insights into the nanostructure, solvation, and dynamics of liquid electrolytes through small-angle X-ray scattering. Adv. Energy Mater. 11, 2002821 (2021).
Leifer, N., Aurbach, D. & Greenbaum, S. G. NMR studies of lithium and sodium battery electrolytes. Prog. Nucl. Magn. Reson. Spectrosc. 142/143, 1–54 (2024).
Kim, T. et al. Applications of voltammetry in lithium ion battery research. J. Electrochem. Sci. Technol. 11, 14–25 (2020).
Hess, S., Wohlfahrt-Mehrens, M. & Wachtler, M. Flammability of Li-ion battery electrolytes: flash point and self-extinguishing time measurements. J. Electrochem. Soc. 162, A3084–A3097 (2015).
Hellweg, L., Beuse, T., Winter, M. & Börner, M. Influence of lithium metal deposition on thermal stability: combined DSC and morphology analysis of cyclic aged lithium metal batteries. J. Electrochem. Soc. 170, 040530 (2023).
Arbizzani, C., Gabrielli, G. & Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 196, 4801–4805 (2011).
Acknowledgements
This Review is the result of a concerted approach within the LILLINT II research project, jointly funded by the US Department of Energy (DOE) and the German Federal Ministry of Research, Technology and Space (BMFTR). A.S. and R.K. acknowledge the financial support of the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technologies Office (VTO), under the Advanced Battery Materials Research (BMR) Program, of the US DOE under contract DE-AC02-05CH11231. R.A., C.-C.S., X.L. and K.A. acknowledge the US DOE, VTO. Argonne National Laboratory is operated by the DOE Office of Science by UChicago Argonne, LLC, under contract DE-AC02-06CH11357. P.B.B. and J.M.S. acknowledge the US DOE through the US–Germany Cooperation on Energy Storage under contract DE-AC02-05CH11357. Computational resources from the Texas A&M University High Performance Research Computing are gratefully acknowledged. The work performed at Pacific Northwest National Laboratory (PNNL) was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, VTO, US DOE and the Advanced BMR Program and the US–Germany Cooperation on Energy Storage with contracts DE-LC-000L072 (C.W.) and DE-AC05-76RL01830 (W.X.). Y.S.-H., C.O.P.-R. and D.W. acknowledge the financial support of the Assistant Secretary for Energy Efficiency and Renewable Energy, VTO, under the Advanced BMR Program of the US DOE under contract DE-AC02-06CH11357, subcontract 9F-60231. J.L. and F.S. thank the National Science Foundation for support under grant 2239690. D.C. and F.S. acknowledge the Assistant Secretary for Energy Efficiency and Renewable Energy, VTO, US DOE through the US–Germany Cooperation on Energy Storage under contract DE-AC02-05CH11357. A.U. and U.K. acknowledge the BMFTR in the framework of LILLINT (03XP0511C). Z.L., D.B., M.Werres., B.H. and A.L. acknowledge financial support within the LILLINT II project (03XP0511E). S.W.-M. and B.v.H. acknowledge financial support within the LILLINT II project (03XP0511D). F.H., R.-A.E, S.B., E.F., I.C.-L., D.W. and M.Winter. acknowledge financial support within the LILLINT II project (13XP0511B).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jianan Wang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weintz, D., Werres, M., Horstmann, B. et al. Nanoengineering of non-aqueous liquid electrolyte solutions for future lithium metal batteries. Nat. Nanotechnol. (2026). https://doi.org/10.1038/s41565-025-02110-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41565-025-02110-z