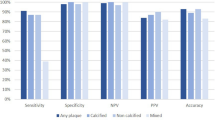
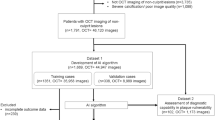
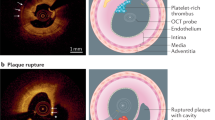
Abstract
Since optical coherence tomography (OCT) was first performed in humans two decades ago, this imaging modality has been widely adopted in research on coronary atherosclerosis and adopted clinically for the optimization of percutaneous coronary intervention. In the past 10 years, substantial advances have been made in the understanding of in vivo vascular biology using OCT. Identification by OCT of culprit plaque pathology could potentially lead to a major shift in the management of patients with acute coronary syndromes. Detection by OCT of healed coronary plaque has been important in our understanding of the mechanisms involved in plaque destabilization and healing with the rapid progression of atherosclerosis. Accurate detection by OCT of sequelae from percutaneous coronary interventions that might be missed by angiography could improve clinical outcomes. In addition, OCT has become an essential diagnostic modality for myocardial infarction with non-obstructive coronary arteries. Insight into neoatherosclerosis from OCT could improve our understanding of the mechanisms of very late stent thrombosis. The appropriate use of OCT depends on accurate interpretation and understanding of the clinical significance of OCT findings. In this Review, we summarize the state of the art in cardiac OCT and facilitate the uniform use of this modality in coronary atherosclerosis. Contributions have been made by clinicians and investigators worldwide with extensive experience in OCT, with the aim that this document will serve as a standard reference for future research and clinical application.
Key points
-
The appropriate use of optical coherence tomography (OCT) depends on the accurate interpretation and understanding of the clinical importance of OCT findings.
-
In vivo diagnosis of plaque erosion with OCT could lead to a major shift in the management of patients with acute coronary syndromes.
-
Detection by OCT of healed coronary plaque is important for understanding the mechanism of plaque destabilization and healing with rapid progression of atherosclerosis.
-
Accurate detection by OCT of findings after percutaneous coronary intervention that could be missed by angiography has the potential to improve clinical outcomes.
-
OCT has become an essential diagnostic modality for patients with myocardial infarction and non-obstructive coronary arteries.
-
Insights from OCT into neoatherosclerosis could improve our understanding of the mechanisms of very late stent thrombosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
18 December 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41569-023-00982-z
References
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Jang, I. K. et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39, 604–609 (2002).
Yabushita, H. et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 106, 1640–1645 (2002).
Tearney, G. J. et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 59, 1058–1072 (2012).
van der Sijde, J. N. et al. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur. Heart J. Cardiovasc. Imaging 18, 467–474 (2017).
Terada, N. et al. Ventricular fibrillation during optical coherence tomography/optical frequency domain imaging — a large single-center experience. Circ. J. 84, 178–185 (2020).
Kubo, T. et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur. Heart J. 38, 3139–3147 (2017).
Ali, Z. A. et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 388, 2618–2628 (2016).
Meneveau, N. et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-st-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 134, 906–917 (2016).
Kubo, T. et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc. Imaging 6, 1095–1104 (2013).
Gerbaud, E. et al. Multi-laboratory inter-institute reproducibility study of IVOCT and IVUS assessments using published consensus document definitions. Eur. Heart J. Cardiovasc. Imaging 17, 756–764 (2016).
Terashima, M. et al. Accuracy and reproducibility of stent-strut thickness determined by optical coherence tomography. J. Invasive Cardiol. 21, 602–605 (2009).
Kini, A. S. et al. Fibrous cap thickness by optical coherence tomography in vivo. J. Am. Coll. Cardiol. 69, 644–657 (2017).
Radu, M. D. et al. Variability in the measurement of minimum fibrous cap thickness and reproducibility of fibroatheroma classification by optical coherence tomography using manual versus semi-automatic assessment. EuroIntervention 12, e987–e997 (2016).
Galon, M. Z. et al. Differences determined by optical coherence tomography volumetric analysis in non-culprit lesion morphology and inflammation in ST-segment elevation myocardial infarction and stable angina pectoris patients. Catheter. Cardiovasc. Interv. 85, E108–E115 (2015).
Tuzcu, E. M. et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 103, 2705–2710 (2001).
Kume, T. et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention 6, 768–772 (2011).
Saita, T. et al. Histopathological validation of optical frequency domain imaging to quantify various types of coronary calcifications. Eur. Heart J. Cardiovasc. Imaging 18, 342–349 (2017).
Ong, D. S. et al. Coronary calcification and plaque vulnerability: an optical coherence tomographic study. Circ. Cardiovasc. Imaging 9, e003929 (2016).
Fujino, A. et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 13, e2182–e2189 (2018).
Raber, L. et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 39, 3281–3300 (2018).
Kawasaki, M. et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J. Am. Coll. Cardiol. 48, 81–88 (2006).
Kato, K. et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ. Cardiovasc. Imaging 5, 433–440 (2012).
Vergallo, R. et al. Prevalence and predictors of multiple coronary plaque ruptures: in vivo 3-vessel optical coherence tomography imaging study. Arterioscler. Thromb. Vasc. Biol. 36, 2229–2238 (2016).
Virmani, R., Burke, A. P., Farb, A. & Kolodgie, F. D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47, C13–C18 (2006).
Yonetsu, T. et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur. Heart J. 32, 1251–1259 (2011).
Tearney, G. J. et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107, 113–119 (2003).
Raber, L. et al. Changes in coronary plaque composition in patients with acute myocardial infarction treated with high-intensity statin therapy (IBIS-4): a serial optical coherence tomography study. JACC Cardiovasc. Imaging 12, 1518–1528 (2018).
Komukai, K. et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J. Am. Coll. Cardiol. 64, 2207–2217 (2014).
Kolodgie, F. D. et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 349, 2316–2325 (2003).
Kume, T. et al. Detection of plaque neovascularization by optical coherence tomography: ex vivo feasibility study and in vivo observation in patients with angina pectoris. J. Invasive Cardiol. 28, 17–22 (2016).
Nishimiya, K. et al. In vivo visualization of adventitial vasa vasorum of the human coronary artery on optical frequency domain imaging. Valid. Study Circ. J. 78, 2516–2518 (2014).
Aoki, T. et al. Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography — animal and human studies. Atherosclerosis 239, 203–208 (2015).
Abela, G. S. & Aziz, K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events — a novel insight into plaque rupture by scanning electron microscopy. Scanning 28, 1–10 (2006).
Crea, F. & Liuzzo, G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 61, 1–11 (2013).
Katayama, Y. et al. Feasibility and clinical significance of in vivo cholesterol crystal detection using optical coherence tomography. Arterioscler. Thromb. Vasc. Biol. 40, 220–229 (2020).
Jinnouchi, H. et al. Detection of cholesterol crystals by optical coherence tomography. EuroIntervention 16, 395–403 (2020).
Kang, S. J. et al. OCT findings in patients with recanalization of organized thrombi in coronary arteries. JACC Cardiovasc. Imaging 5, 725–732 (2012).
Souteyrand, G. et al. Diagnosis and management of spontaneously recanalized coronary thrombus guided by optical coherence tomography — lessons from the French “Lotus Root” Registry. Circ. J. 82, 783–790 (2018).
Prati, F. et al. Expert review document. Part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 33, 2513–2520 (2012).
Kajander, O. A. et al. Feasibility and repeatability of optical coherence tomography measurements of pre-stent thrombus burden in patients with STEMI treated with primary PCI. Eur. Heart J. Cardiovasc. Imaging 16, 96–107 (2015).
Burke, A. P. et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 103, 934–940 (2001).
Mann, J. & Davies, M. J. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart 82, 265–268 (1999).
Otsuka, F., Joner, M., Prati, F., Virmani, R. & Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 11, 379–389 (2014).
Vergallo, R. & Crea, F. Atherosclerotic plaque healing. N. Engl. J. Med. 383, 846–857 (2020).
Shimokado, A. et al. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis 275, 35–42 (2018).
Hoshino, M. et al. Optical coherence tomographic features of unstable coronary lesions corresponding to histopathological intraplaque hemorrhage evaluated by directional coronary atherectomy specimens. JACC Cardiovasc. Interv. 11, 1414–1415 (2018).
Antuna, P. et al. Diagnosis of intraplaque hemorrhage by high-definition intravascular ultrasound and optical coherence tomography. JACC Cardiovasc. Interv. 13, 1960–1962 (2020).
Pollack, A., Nazif, T., Mancini, D. & Weisz, G. Detection and imaging of cardiac allograft vasculopathy. JACC Cardiovasc. Imaging 6, 613–623 (2013).
Cassar, A. et al. Coronary atherosclerosis with vulnerable plaque and complicated lesions in transplant recipients: new insight into cardiac allograft vasculopathy by optical coherence tomography. Eur. Heart J. 34, 2610–2617 (2013).
Dong, L. et al. Optical coherence tomographic evaluation of transplant coronary artery vasculopathy with correlation to cellular rejection. Circ. Cardiovasc. Interv. 7, 199–206 (2014).
Shan, P. et al. Comparison between cardiac allograft vasculopathy and native coronary atherosclerosis by optical coherence tomography. Am. J. Cardiol. 117, 1361–1368 (2016).
Ichibori, Y. et al. Optical coherence tomography and intravascular ultrasound evaluation of cardiac allograft vasculopathy with and without intimal neovascularization. Eur. Heart J. Cardiovasc. Imaging 17, 51–58 (2016).
Clemmensen, T. S. et al. Layered fibrotic plaques are the predominant component in cardiac allograft vasculopathy: systematic findings and risk stratification by OCT. JACC Cardiovasc. Imaging 10, 773–784 (2017).
Clemmensen, T. S. et al. Detection of early changes in the coronary artery microstructure after heart transplantation: a prospective optical coherence tomography study. J. Heart Lung Transpl. 37, 486–495 (2018).
Gerbaud, E. et al. Plaque burden can be assessed using intravascular optical coherence tomography and a dedicated automated processing algorithm: a comparison study with intravascular ultrasound. Eur. Heart J. Cardiovasc. Imaging 21, 640–652 (2019).
Ramasamy, A. et al. Efficacy and reproducibility of attenuation-compensated optical coherence tomography for assessing external elastic membrane border and plaque composition in native and stented segments- an in vivo and histology-based study. Circ. J. 84, 91–100 (2019).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Partida, R. A., Libby, P., Crea, F. & Jang, I. K. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur. Heart J. 39, 2070–2076 (2018).
Arbustini, E. et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 82, 269–272 (1999).
Higuma, T. et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with st-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc. Interv. 8, 1166–1176 (2015).
Jia, H. et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 62, 1748–1758 (2013).
Prati, F. et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc. Imaging 6, 283–287 (2013).
Jia, H. et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 38, 792–800 (2017).
Xing, L. et al. EROSION study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): a 1-year follow-up report. Circ. Cardiovasc. Interv. 10, e005860 (2017).
Luping, H. et al. Predictors of non-stenting strategy for acute coronary syndrome caused by plaque erosion: 4-year outcomes of the EROSION study. EuroIntervention 17, 497–505 (2020).
Combaret, N. et al. Management of ST-elevation myocardial infarction in young patients by limiting implantation of durable intracoronary devices and guided by optical frequency domain imaging: “proof of concept” study. EuroIntervention 13, 397–406 (2017).
Torii, S. et al. Eruptive calcified nodules as a potential mechanism of acute coronary thrombosis and sudden death. J. Am. Coll. Cardiol. 77, 1599–1611 (2021).
Kobayashi, N. et al. Features and outcomes of patients with calcified nodules at culprit lesions of acute coronary syndrome: an optical coherence tomography study. Cardiology 139, 90–100 (2018).
Sugiyama, T. et al. Calcified plaques in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 12, 531–540 (2019).
Saw, J. et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur. Heart J. 40, 1188–1197 (2019).
Saw, J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter. Cardiovasc. Interv. 84, 1115–1122 (2014).
Tamis-Holland, J. E. et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 139, e891–e908 (2019).
Gerbaud, E. et al. OCT and CMR for the diagnosis of patients presenting with MINOCA and Suspected epicardial causes. JACC Cardiovasc. Imaging 13, 2619–2631 (2020).
Reynolds, H. R. et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 143, 624–640 (2021).
Xing, L. et al. Clinical significance of lipid-rich plaque detected by optical coherence tomography: a 4-year follow-up study. J. Am. Coll. Cardiol. 69, 2502–2513 (2017).
Jang, I. K. Pursuit for the detection of vulnerable plaque. Eur. Heart J. 41, 392–393 (2020).
Prati, F. et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur. Heart J. 41, 383–391 (2019).
Kubo, T. et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging https://doi.org/10.1093/ehjci/jeab028 (2021).
Ross, R. The pathogenesis of atherosclerosis — an update. N. Engl. J. Med. 314, 488–500 (1986).
Jang, I. K. Plaque progression slow linear or rapid stepwise? Circ. Cardiovasc. Imaging 314, 488–500 (2017).
Uemura, S. et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 33, 78–85 (2012).
Yamamoto, M. H. et al. Serial 3-vessel optical coherence tomography and intravascular ultrasound analysis of changing morphologies associated with lesion progression in patients with stable angina pectoris. Circ. Cardiovasc. Imaging 10, e006347 (2017).
Araki, M. et al. Predictors of rapid plaque progression: an optical coherence tomography study. JACC Cardiovasc. Imaging 14, 1628–1638 (2021).
Nicholls, S. J. et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 316, 2373–2384 (2016).
Hattori, K. et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc. Imaging 5, 169–177 (2012).
Yano, H., Horinaka, S. & Ishimitsu, T. Effect of evolocumab therapy on coronary fibrous cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. J. Cardiol. 75, 289–295 (2019).
Nicholls, S. J. et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 11, 120–129 (2021).
Wijns, W. et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur. Heart J. 36, 3346–3355 (2015).
Kubo, T. et al. Superficial calcium fracture after PCI as assessed by OCT. JACC Cardiovasc. Imaging 8, 1228–1229 (2015).
Imola, F. et al. Association between proximal stent edge positioning on atherosclerotic plaques containing lipid pools and postprocedural myocardial infarction (from the CLI-POOL Study). Am. J. Cardiol. 111, 526–531 (2013).
Ino, Y. et al. Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ. Cardiovasc. Interv. 9, e004231 (2016).
Belguidoum, S. et al. Relationship between stent expansion and fractional flow reserve after percutaneous coronary intervention: a post hoc analysis of the DOCTORS trial. EuroIntervention 17, e132–e139 (2021).
Prati, F. et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc. Imaging 8, 1297–1305 (2015).
Soeda, T. et al. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation 132, 1020–1029 (2015).
Antonsen, L. et al. Optical coherence tomography guided percutaneous coronary intervention with Nobori stent implantation in patients with non-st-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ. Cardiovasc. Interv. 8, e002446 (2015).
Adriaenssens, T. et al. Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation 136, 1007–1021 (2017).
Souteyrand, G. et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur. Heart J. 37, 1208–1216 (2016).
Prati, F. et al. Clinical impact of suboptimal stenting and residual intrastent plaque/thrombus protrusion in patients with acute coronary syndrome: the CLI-OPCI ACS substudy (Centro per la Lotta Contro L’Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ. Cardiovasc. Interv. 9, e003726 (2016).
Kawamori, H. et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur. Heart J. Cardiovasc. Imaging 14, 865–875 (2013).
Radu, M. D. et al. Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention 9, 1085–1094 (2014).
Prati, F. et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 8, 823–829 (2012).
Sheth, T. N. et al. Optical coherence tomography-guided percutaneous coronary intervention in st-segment-elevation myocardial infarction: a prospective propensity-matched cohort of the thrombectomy versus percutaneous coronary intervention alone trial. Circ. Cardiovasc. Interv. 9, e003414 (2016).
Iannaccone, M. et al. Impact of an optical coherence tomography guided approach in acute coronary syndromes: a propensity matched analysis from the international FORMIDABLE-CARDIOGROUP IV and USZ registry. Catheter. Cardiovasc. Interv. 90, E46–E52 (2017).
Lee, S. Y. et al. early strut coverage in patients receiving drug-eluting stents and its implications for dual antiplatelet therapy: a randomized trial. JACC Cardiovasc. Imaging 11, 1810–1819 (2018).
Jones, D. A. et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the Pan-London PCI Cohort. JACC Cardiovasc. Interv. 11, 1313–1321 (2018).
Ali, Z. A. et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: OPTIMIZE PCI trial. EuroIntervention 16, 1085–1091 (2021).
Ali, Z. et al. Optical coherence tomography-guided coronary stent implantation compared to angiography: a multicentre randomised trial in PCI - design and rationale of ILUMIEN IV: OPTIMAL PCI. EuroIntervention 16, 1092–1099 (2021).
Kubo, T. et al. Comparison between optical coherence tomography guidance and angiography guidance in percutaneous coronary intervention (COCOA): study protocol for a randomized controlled trial. J. Cardiol. 72, 170–175 (2018).
Holm, N. R. et al. Rational and design of the European randomized Optical Coherence Tomography Optimized Bifurcation Event Reduction Trial (OCTOBER). Am. Heart J. 205, 97–109 (2018).
Buccheri, S. et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc. Interv. 10, 2488–2498 (2017).
Bezerra, H. G. et al. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc. Interv. 6, 228–236 (2013).
Okamura, T. et al. First-in-man evaluation of intravascular optical frequency domain imaging (OFDI) of Terumo: a comparison with intravascular ultrasound and quantitative coronary angiography. EuroIntervention 6, 1037–1045 (2011).
Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165 (2019).
Fihn, S. D. et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 60, e44–e164 (2012).
Patel, M. R. et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 69, 2212–2241 (2017).
Burzotta, F. et al. Fractional flow reserve or optical coherence tomography to guide management of angiographically intermediate coronary stenosis: a single-center trial. JACC Cardiovasc. Interv. 13, 49–58 (2020).
Kennedy, M. W. et al. Combined optical coherence tomography morphologic and fractional flow reserve hemodynamic assessment of non- culprit lesions to better predict adverse event outcomes in diabetes mellitus patients: COMBINE (OCT-FFR) prospective study. Rationale and design. Cardiovasc. Diabetol. 15, 144 (2016).
Burzotta, F. et al. Correlation between frequency-domain optical coherence tomography and fractional flow reserve in angiographically-intermediate coronary lesions. Int. J. Cardiol. 253, 55–60 (2018).
Usui, E. et al. Efficacy of optical coherence tomography-derived morphometric assessment in predicting the physiological significance of coronary stenosis: head-to-head comparison with intravascular ultrasound. EuroIntervention 13, e2210–e2218 (2018).
Ramasamy, A. et al. Optical coherence tomography enables more accurate detection of functionally significant intermediate non-left main coronary artery stenoses than intravascular ultrasound: a meta-analysis of 6919 patients and 7537 lesions. Int. J. Cardiol. 301, 226–234 (2020).
Huang, J. et al. Diagnostic performance of intracoronary optical coherence tomography-based versus angiography-based fractional flow reserve for the evaluation of coronary lesions. EuroIntervention 16, 568–576 (2020).
Ding, D. et al. Optical Flow ratio for assessing stenting result and physiological significance of residual disease. EuroIntervention 17, e989–e998 (2021).
Onuma, Y. et al. A randomized trial evaluating online 3-dimensional optical frequency domain imaging-guided percutaneous coronary intervention in bifurcation lesions. Circ. Cardiovasc. Interv. 13, e009183 (2020).
Amabile, N. et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: the LEMON study. EuroIntervention 17, e124–e131 (2021).
Onuma, Y. et al. Joint consensus on the use of OCT in coronary bifurcation lesions by the European and Japanese bifurcation clubs. EuroIntervention 14, e1568–e1577 (2019).
Templin, C. et al. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur. Heart J. 31, 1792–1801 (2010).
Jinnouchi, H. et al. Healthy strut coverage after coronary stent implantation: an ex vivo human autopsy study. Circ. Cardiovasc. Interv. 13, e008869 (2020).
Radu, M. D. et al. Coronary evaginations are associated with positive vessel remodelling and are nearly absent following implantation of newer-generation drug-eluting stents: an optical coherence tomography and intravascular ultrasound study. Eur. Heart J. 35, 795–807 (2014).
Yamamoto, E. et al. Dynamic neointimal pattern after drug-eluting stent implantation defined by optical coherence tomography. Coron. Artery Dis. 28, 557–563 (2017).
Lutter, C. et al. Histopathological differential diagnosis of optical coherence tomographic image interpretation after stenting. JACC Cardiovasc. Interv. 9, 2511–2523 (2016).
Xhepa, E. et al. Clinical outcomes by optical characteristics of neointima and treatment modality in patients with coronary in-stent restenosis. EuroIntervention 17, e388–e395 (2020).
Madhavan, M. V. et al. Stent-related adverse events >1 year after percutaneous coronary intervention. J. Am. Coll. Cardiol. 75, 590–604 (2020).
Takano, M. et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J. Am. Coll. Cardiol. 55, 26–32 (2009).
Nakazawa, G. et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 57, 1314–1322 (2011).
Taniwaki, M. et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 133, 650–660 (2016).
Koskinas, K. C. et al. Current use of intracoronary imaging in interventional practice — results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention 14, e475–e484 (2018).
Zhou, J. et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a health economic analysis. Circ. Cardiovasc. Qual. Outcomes 14, e006789 (2021).
Alberti, A. et al. Understanding the economic impact of intravascular ultrasound (IVUS). Eur. J. Health Econ. 17, 185–193 (2016).
Min, H. S. et al. Detection of optical coherence tomography-defined thin-cap fibroatheroma in the coronary artery using deep learning. EuroIntervention 16, 404–412 (2019).
Chu, M. et al. Artificial intelligence and optical coherence tomography for the automatic characterisation of human atherosclerotic plaques. EuroIntervention 17, 41–50 (2021).
Shibutani, H. et al. Automated classification of coronary atherosclerotic plaque in optical frequency domain imaging based on deep learning. Atherosclerosis 328, 100–105 (2021).
Yin, J. et al. Integrated intravascular optical coherence tomography ultrasound imaging system. J. Biomed. Opt. 15, 010512 (2010).
Fard, A. M. et al. Optical coherence tomography — near infrared spectroscopy system and catheter for intravascular imaging. Opt. Express 21, 30849–30858 (2013).
Yoo, H. et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 17, 1680–1684 (2011).
Park, J. et al. A dual-modality optical coherence tomography and fluorescence lifetime imaging microscopy system for simultaneous morphological and biochemical tissue characterization. Biomed. Opt. Express 1, 186–200 (2010).
Liu, L. et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat. Med. 17, 1010–1014 (2011).
de Boer, J. F., Hitzenberger, C. K. & Yasuno, Y. Polarization sensitive optical coherence tomography-a review [Invited]. Biomed. Opt. Express 8, 1838–1873 (2017).
Tearney, G. J. et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science 276, 2037–2039 (1997).
Yun, S., Tearney, G., de Boer, J., Iftimia, N. & Bouma, B. High-speed optical frequency-domain imaging. Opt. Express 11, 2953–2963 (2003).
Kim, J. S. et al. Neointimal patterns obtained by optical coherence tomography correlate with specific histological components and neointimal proliferation in a swine model of restenosis. Eur. Heart J. Cardiovasc. Imaging 15, 292–298 (2014).
Gonzalo, N. et al. Optical coherence tomography patterns of stent restenosis. Am. Heart J. 158, 284–293 (2009).
Acknowledgements
I.-K.J.’s research is supported by Mrs Gillian Gray through the Allan Gray Fellowship Fund in Cardiology.
Author information
Authors and Affiliations
Contributions
M.A. and I.-K.J. discussed the content of the article and wrote the manuscript. All the authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.L.D. is a consultant to Baim Clinical Research Institute, Boston Scientific, Cardiovascular Research Foundation and Medtronic, and has research grants from Boston Scientific and Medtronic. S.U. received educational grants from Abbott Vascular Japan. J.-S.K. received proctoring fees from Abbott Vascular. C.D.M. received research grants (to the institution) from AMGEN, Behring, Boston Scientific, Chiesi, Daiichi-Sankyo, Edwards, Medtronic, and Shockwave Volcano-Philips and speaker fees from Abbott and Shockwave. T.W.J. received consultancy and speaker fees from Abbott Vascular & Terumo. G.G. received consultant fees from Abbott Vascular and Infraredx and research grants from Abbott Vascular, Amgen and Infraredx. M.J. received personal fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Edwards, OrbusNeich, Recor, and Shockwave and grants from Amgen, Boston Scientific, Cardiac Dimensions, Edwards and Infraredx. N.R.H. received speaker fees from Terumo, research grants and speaker fees from Abbott and Reva Medical, and research grants from B. Braun, Biosensors, Boston Scientific and Medis Medical Imaging. W.W. received institutional research grants and honoraria from MicroPort (steering Committee TARGET AC trial); is co-founder of Argonauts, an innovation facilitator; and is medical adviser to Rede Optimus Research and Corrib Core Laboratory, NUI Galway. T.Adriaenssens received educational grants from Abbott Vascular. H.N. received speaker honoraria and research grants from Abbott Vascular. N.A. received proctoring and consulting fees from Abbott Vascular and Boston Scientific, consulting fees from Shockwave Medical, and institutional research grants from Abbott Vascular. G.S. received consulting fees from Abbott Medical, Boston Scientific, Medtronic, Shockwave and Terumo. E.G. is a consultant for Abbott Vascular and Terumo. N.G. received speaker and consultant fees from Abbott and speaker fees from Boston Scientific. G.J.T. receives sponsored research funding from AstraZeneca, Canon, CN USA Biotech Holdings, and VivoLight and catheter materials from Terumo. G.J.T. has a financial/fiduciary interest in SpectraWave, a company developing an OCT–NIRS intracoronary imaging system and catheter; this financial/fiduciary interest was reviewed and is managed by the Massachusetts General Hospital and Mass General Brigham HealthCare in accordance with their conflict-of-interest policies. G.J.T. (Canon, Spectrawave and Terumo) has the right to receive royalties from licensing arrangements. B.B. has OCT patents assigned to Massachusetts General Hospital and licensed to Terumo. A.D.A. received research grants from Amgen and Philips Healthcare. G.S.M. received honoraria from Abiomed, Boston Scientific, Medtronic and Philips/Volcano, and has equity in SpectraWave. G.W.S. received speaker honoraria from Cook, Infraredx and Terumo; is a consultant to Ablative Solutions, Abiomed, Ancora, Cardiomech, CorFlow, Elucid Bio, Gore, HeartFlow, MAIA Pharmaceuticals, Miracor, Neovasc, Occlutech, Reva, Robocath, Shockwave, TherOx, Valfix, Vascular Dynamics, Vectorious and V-Wave; and has equity/options from Ancora, Applied Therapeutics, Aria, Biostar family of funds, Cagent, Cardiac Success, MedFocus family of funds, Orchestra Biomed, SpectraWave and Valfix. L.R. received grants to the institution from Abbott, Biotronik, Boston Scientific, Heartflow, Sanofi, and Regeneron and speaker/consultation fees from Abbott, Amgen, AstraZeneca, Canon, Occlutec, Sanofi and Vifor. T.S. received research grants from Abbott Medical Japan. B.P.Y. received research grants and speaker honorarium from Abbott Vascular. L.K.M. received departmental grants from Abbott. H.R. received donations for research from Abbott Vascular, BioTelemetry and Siemens. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, Sanofi-Regeneron. P.L. is a member of scientific advisory board for Amgen, Caristo, Cartesian, Corvidia Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Medimmune, Novartis, Olatec Therapeutics, PlaqueTec and XBiotech. P.L.’s laboratory has received research funding in the past 2 years from Novartis. P.L. is on the Board of Directors of XBiotech. P.L. has a financial interest in Xbiotech, a company developing therapeutic human antibodies. P.L.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. G.W. is a member of the medical advisory board for Filterlex, Intratech, Microbot and Trisol, and received equity from Filterlex, Intratech and Microbot and consulting fees from Cuspa, Filterlex, Intratech, Magenta and Microbot. T.G. received speaker’s honoraria and research support from Abbot Vascular. K.T. received research grants from Medtronic, and is proctor for Abbott and Medtronic. T.Y. received an endowment from Abbott Vascular Japan, Boston Scientific Japan, Japan Lifeline, Takeyama KK and WIN International. Y.M. received an honorarium and consulting fee from Abbott. R.Vergallo received speaker fees from Abbott. E.A. received grants from the Ministry of Health to the National IRCCS Cardiology Network (RCR-2019-23669116-001 and RCR-2020-23670065) and from FRRB grant CP_14/2018, INTESTRAT-CAD, Lombardia Region, Italy. H.M.G.-G. received institutional grant support from Abbott, Biotronik, Boston Scientific, CorFlow, Medtronic, Neovasc, Philips and Shockwave. Z.A. received institutional grants from Abbott Vascular and Cardiovascular Systems to Columbia University and Cardiovascular Research Foundation; honoraria from Amgen, AstraZeneca and Boston Scientific; and equity from Shockwave. A.V.F. and R.Virmani received institutional research support from NIH (HL141425), Leducq Foundation Grant; 480 Biomedical, 4C Medical, 4Tech, Abbott, Accumedical, Amgen, Biosensors, Boston Scientific, Cardiac Implants, Celonova, Claret Medical, Concept Medical, Cook, CSI, DuNing, Edwards Lifesciences, Emboline, Endotronix, Envision Scientific, Lutonix/Bard, Gateway, Lifetech, Limflo, MedAlliance, Medtronic, Mercator, Merill, Microport Medical, Microvention, MitraAlign, Mitra Assist, NAMSA, Nanova, Neovasc, NIPRO, Novogate, Occulotech, OrbusNeich Medical, Phenox, Profusa, Protembis, Qool, Recor, Senseonics, Shockwave, Sinomed, Spectranetics, Surmodics, Symic, Vesper, W.L. Gore and Xeltis. A.V.F. received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, CSI, Lutonix Bard, Sinomed and Terumo; and is a consultant to Amgen, Abbott Vascular, Boston Scientific, Celonova, Cook Medical, Lutonix Bard and Sinomed. R.Virmani received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, Cordis, CSI, Lutonix Bard, Medtronic, OrbusNeich Medical, CeloNova, SINO Medical Technology, ReCore, Spectranetics, Terumo, and W. L. Gore and is a consultant for Abbott Vascular, Boston Scientific, Celonova, Cook Medical, Cordis, CSI, Edwards Lifesciences, Lutonix Bard, Medtronic, OrbusNeich Medical, ReCore, Sinomededical Technology, Spectranetics, Surmodics, Terumo, W. L. Gore and Xeltis. T.K. received personal fees from Abbott Japan. K.H. received remuneration for lectures from Terumo, Abbott Vascular and Boston Scientific Japan. T.Akasaka received research grants from Abbott Vascular Japan, Nipro, and Terumo and is a medical adviser for Terumo. A.L. received consultant fees from Volcano and Philips. E.R. is a member of the medical advisory board for Zed Medical and a clinical adviser for Kaminari Medical. B.Y. received research grants from the National Key R&D Program of China (2016YFC1301103) and the National Natural Science Foundation of China (81827806). F.C. received personal fees from Amgen, AstraZeneca, BMS and Servier, and other fees from GlyCardial Diagnostics. J.F. has financial interests in Optovue, receives royalties from intellectual property owned by MIT and licensed to Optovue and receives research support from the NIH and Topcon. I.-K.J. received educational grants from Abbott Vascular and consulting fees from Mitobridge and Svelte. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Antonio Colombo, Patrick Serruys and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of authors and their affiliations appears at the end of the paper.
Supplementary information
Glossary
- Axial resolution
-
The minimum distance between two objects that can be resolved along the axial dimension.
- Near-infrared
-
Light with wavelengths extending from 0.78 µm to 2.50 µm.
- Cross-sectional images
-
The traditional mode for optical coherence tomography (OCT) images that displays a cross-section of the vessel with a circular shape.
- Penetration depth
-
The depth within a tissue or object at which an OCT image signal has been attenuated (via scattering or absorption) to a level indistinguishable from the background noise.
- L-mode
-
The longitudinal mode represents an OCT image along the longitudinal dimension at a particular rotational angle.
- Co-registration
-
The process of registering two or more images so that they can be viewed and analysed together.
- Backscattered light
-
The reflection of light back in the direction from which it came; usually a diffuse reflection due to scattering as opposed to specular reflection as from a mirror.
- Leading edge
-
The first edge in an object that is encountered along a vector that is pointing away from the catheter; by contrast, the trailing edge is the last edge that is encountered.
- Z-offset
-
Slight variations in optical path length within the catheter, which can be adjusted using the catheter diameter within an image as a reference.
- Refractive index
-
A property of a material that governs the speed of light through that material.
- Centroid
-
The arithmetic mean position of all the points in the figure; also known as the centre of mass.
- Adluminal
-
Near or towards the lumen.
- Pullback rate
-
The speed at which an OCT catheter is pulled back during imaging.
- Frame rate
-
The number of images captured per second.
- Attenuation
-
Loss of light due to scattering and/or absorption by flushing media, blood or tissue that results in a weaker OCT signal.
- Speckle
-
The grainy pattern that appears in OCT images because of the interference of waves with random phase.
- Abluminal
-
Away from the lumen.
- Computational fluid dynamics
-
A branch of fluid mechanics that uses numerical analysis and data structures to analyse and solve problems that involve fluid flows.
- Contour
-
The boundary of a certain object, such as the lumen, stent or plaque component.
- Deep learning
-
A class of machine learning algorithm that uses multiple layers to progressively extract higher-level features from the raw input.
- Fluorescence
-
The emission of light by a substance that has absorbed light or other electromagnetic radiation.
- Polarization
-
A property of light described by the magnitude, orientation and precession of its electric field.
- Birefringence
-
A phenomenon exhibited by a material in which light travelling through the material is divided into two beams of different polarizations.
- Depolarization
-
When the incident beam is fully linearly or circularly polarized, the scattered light can become partially polarized or even totally unpolarized.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araki, M., Park, SJ., Dauerman, H.L. et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat Rev Cardiol 19, 684–703 (2022). https://doi.org/10.1038/s41569-022-00687-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-022-00687-9
This article is cited by
-
Association between triglyceride-glucose index and OCT-defined high-risk coronary plaques in stable angina
BMC Cardiovascular Disorders (2026)
-
The prognostic value of atherogenic index of plasma and thin-cap fibroatheroma among patients with STEMI: an optical coherence tomography prospective cohort study of real world
Cardiovascular Diabetology (2025)
-
Thrombus rooting in the pulmonary arteriovenous fistula in a patient with cryptogenic stroke, a case report
BMC Neurology (2025)
-
Inflammation mediates the relationship between cardiometabolic index and vulnerable plaque in patients with acute coronary syndrome
Lipids in Health and Disease (2025)
-
Mapping the distribution of radial artery atherosclerosis by optical coherence tomography
BMC Medical Imaging (2025)