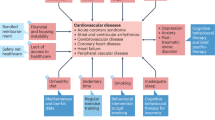
Abstract
The majority of cardiovascular randomized controlled trials (RCTs) test interventions in selected patient populations under explicitly protocol-defined settings. Although these ‘explanatory’ trial designs optimize conditions to test the efficacy and safety of an intervention, they limit the generalizability of trial findings in broader clinical settings. The concept of ‘pragmatism’ in RCTs addresses this concern by providing counterbalance to the more idealized situation underpinning explanatory RCTs and optimizing effectiveness over efficacy. The central tenets of pragmatism in RCTs are to test interventions in routine clinical settings, with patients who are representative of broad clinical practice, and to reduce the burden on investigators and participants by minimizing the number of trial visits and the intensity of trial-based testing. Pragmatic evaluation of interventions is particularly important in cardiovascular diseases, where the risk of death among patients has remained fairly stable over the past few decades despite the development of new therapeutic interventions. Pragmatic RCTs can help to reveal the ‘real-world’ effectiveness of therapeutic interventions and elucidate barriers to their implementation. In this Review, we discuss the attributes of pragmatism in RCT design, conduct and interpretation as well as the general need for increased pragmatism in cardiovascular RCTs. We also summarize current challenges and potential solutions to the implementation of pragmatism in RCTs and highlight selected ongoing and completed cardiovascular RCTs with pragmatic trial designs.
Key points
-
Most cardiovascular randomized controlled trials (RCTs) conducted to date have been ‘explanatory’, that is, designed to study the intervention in optimized conditions with selected patient populations and frequent protocolized assessments.
-
Although explanatory RCT designs increase validity, they limit the generalizability of trial findings, whereas a ‘pragmatic’ approach to RCTs yields findings more relevant to real-world practice.
-
In pragmatic RCTs, interventions are tested in patients who are broadly representative of the condition being studied, and the study is aligned with routine clinical care to reduce costs and organizational burden.
-
Although pragmatic RCTs tend to attenuate estimates of treatment effects, they do provide a more realistic understanding of population-level effectiveness and costs than explanatory trials.
-
Pragmatic trials can highlight barriers to the implementation of therapies and are better suited than explanatory RCTs to assessing the effects of implementation strategies and health-care policies at the population level.
-
Widespread implementation of pragmatic trials would require the development of technological infrastructure to collect and share data as well as regulatory guidelines amenable to findings derived from routinely collected data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhu, J. W. et al. Global representation of heart failure clinical trial leaders, collaborators, and enrolled participants: a bibliometric review 2000–2020. Eur. Heart J. Qual. Care Clin. Outcomes https://doi.org/10.1093/ehjqcco/qcab058 (2021).
Schwartz, D. & Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Chronic Dis. 20, 637–648 (1967).
Merali, Z. & Wilson, J. R. Explanatory versus pragmatic trials: an essential concept in study design and interpretation. Clin. Spine Surg. 30, 404–406 (2017).
Ford, I. & Norrie, J. Pragmatic Trials. N. Engl. J. Med. 375, 454–463 (2016).
Van Spall, H. G. C., Averbuch, T., Damman, K. & Voors, A. A. Risk and risk reduction in trials of heart failure with reduced ejection fraction: absolute or relative? Eur. J. Heart Fail. 23, 1437–1444 (2021).
Ferreira, J. P. et al. Natriuretic peptides, 6-min walk test, and quality-of-life questionnaires as clinically meaningful endpoints in HF trials. J. Am. Coll. Cardiol. 68, 2690–2707 (2016).
Greene, S. J. et al. Reassessing the role of surrogate end points in drug development for heart failure. Circulation 138, 1039–1053 (2018).
Thorpe, K. E. et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J. Clin. Epidemiol. 62, 464–475 (2009).
Loudon, K. et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 350, h2147 (2015).
Farrow, L., Gardner, W. T., Ablett, A. D., Kutuzov, V. & Johnstone, A. A review of trauma and orthopaedic randomised clinical trials published in high-impact general medical journals. Eur. J. Orthop. Surg. Traumatol. https://doi.org/10.1007/s00590-021-03137-3 (2021).
Hohenschurz-Schmidt, D. et al. Pragmatic trials of pain therapies: a systematic review of methods. Pain 163, 21–46 (2022).
Burnett, H. et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. Circ. Heart Fail. 10, e003529 (2017).
Bassi, N. S., Ziaeian, B., Yancy, C. W. & Fonarow, G. C. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol. 5, 948–951 (2020).
Bardy, G. H. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 352, 225–237 (2005).
Cleland, J. G. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 352, 1539–1549 (2005).
Roccaforte, R., Demers, C., Baldassarre, F., Teo, K. K. & Yusuf, S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients: a meta-analysis. Eur. J. Heart Fail. 7, 1133–1144 (2005).
Blue, L. et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 323, 715–718 (2001).
Taylor, C. J. et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ 364, l223 (2019).
Gerber, Y. et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 175, 996–1004 (2015).
Taylor, C. J. et al. Survival following a diagnosis of heart failure in primary care. Fam. Pract. 34, 161–168 (2017).
Conrad, N. et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol. 4, 1102–1111 (2019).
Coffey, S., Cox, B. & Williams, M. J. Lack of progress in valvular heart disease in the pre-transcatheter aortic valve replacement era: increasing deaths and minimal change in mortality rate over the past three decades. Am. Heart J. 167, 562–567.e2 (2014).
Vinter, N. et al. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ 370, m2724 (2020).
Tanaka, Y. et al. Trends in cardiovascular mortality related to atrial fibrillation in the United States, 2011 to 2018. J. Am. Heart Assoc. 10, e020163 (2021).
Greene Stephen, J. et al. Medical therapy for heart failure with reduced ejection fraction. J. Am. Coll. Cardiol. 72, 351–366 (2018).
Fiuzat, M. et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 5, 757–764 (2020).
Keramida, K. & Filippatos, G. Heart failure guidelines implementation: lifting barriers using registries and networks. Anatol. J. Cardiol. 24, 41–42 (2020).
Usman, M. S., Pitt, B. & Butler, J. Target trial emulations: bridging the gap between clinical trial and real-world data. Eur. J. Heart Fail. 23, 1708–1711 (2021).
Sedgwick, P. Bias in observational study designs: prospective cohort studies. BMJ 349, g7731 (2014).
Fanaroff, A. C. et al. Randomized trials versus common sense and clinical observation: JACC review topic of the week. J. Am. Coll. Cardiol. 76, 580–589 (2020).
Whitbeck, M. G. et al. Increased mortality among patients taking digoxin — analysis from the AFFIRM study. Eur. Heart J. 34, 1481–1488 (2013).
Gheorghiade, M. et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur. Heart J. 34, 1489–1497 (2013).
Van Spall, H. G., Toren, A., Kiss, A. & Fowler, R. A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 297, 1233–1240 (2007).
Nanna, M. G., Chen, S. T., Nelson, A. J., Navar, A. M. & Peterson, E. D. Representation of older adults in cardiovascular disease trials since the inclusion across the lifespan policy. JAMA Intern. Med. 180, 1531–1533 (2020).
Jadad, A. R., To, M. J., Emara, M. & Jones, J. Consideration of multiple chronic diseases in randomized controlled trials. JAMA 306, 2670–2672 (2011).
Arnett, D. K. et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions. Circulation 130, 1662–1667 (2014).
Whitelaw, S. et al. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur. J. Heart Fail. 23, 15–24 (2021).
Obadia, J.-F. et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N. Engl. J. Med. 379, 2297–2306 (2018).
Stone, G. W. et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 379, 2307–2318 (2018).
McMurray, J. J. V. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Mann, D. L. et al. Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the LIFE trial. JACC Heart Fail. 8, 789–799 (2020).
Samman Tahhan, A. et al. Design elements and enrollment patterns of contemporary trials in heart failure with preserved ejection fraction. JACC Heart Fail. 6, 714–717 (2018).
Moyé, L. Clinical trials in cardiology. Circ. Res. 114, 28–31 (2014).
Williams, R. J., Tse, T., DiPiazza, K. & Zarin, D. A. Terminated trials in the clinicaltrials.gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS ONE 10, e0127242 (2015).
Wei, S. et al. Factors associated with racial and ethnic diversity among heart failure trial participants: a systematic bibliometric review. Circ. Heart Fail. 15, e008685 (2022).
Khan, M. S. et al. Ten‐year trends in enrollment of women and minorities in pivotal trials supporting recent us food and drug administration approval of novel cardiometabolic drugs. J. Am. Heart Assoc. 9, e015594 (2020).
Greene, S. J. et al. Representativeness of a heart failure trial by race and sex: results from ASCEND-HF and GWTG-HF. JACC Heart Fail. 7, 980–992 (2019).
Bernabe-Ortiz, A. et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat. Med. 26, 374–378 (2020).
Felker, G. M. et al. Effect of natriuretic peptide–guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 318, 713–720 (2017).
Beck-da-Silva, L., de Bold, A., Fraser, M., Williams, K. & Haddad, H. BNP-guided therapy not better than expert’s clinical assessment for beta-blocker titration in patients with heart failure. Congest. Heart Fail. 11, 248–253 (2005).
McCord, K. A. et al. Treatment effects in randomised trials using routinely collected data for outcome assessment versus traditional trials: meta-research study. BMJ 372, n450 (2021).
Breckenridge, A. et al. Poor medication adherence in clinical trials: consequences and solutions. Nat. Rev. Drug Discov. 16, 149–150 (2017).
Laursen, D. R. T., Paludan-Müller, A. S. & Hróbjartsson, A. Randomized clinical trials with run-in periods: frequency, characteristics and reporting. Clin. Epidemiol. 11, 169–184 (2019).
Verberk, W. J. et al. Home versus Office Blood Pressure Measurements: Reduction of Unnecessary Rreatment Study: rationale and study design of the HOMERUS trial. Blood Press. 12, 326–333 (2003).
van Onzenoort, H. A. W. et al. Participation in a clinical trial enhances adherence and persistence to treatment. Hypertension 58, 573–578 (2011).
Vonbank, A. et al. Reasons for disparity in statin adherence rates between clinical trials and real-world observations: a review. Eur. Heart J. Cardiovasc. Pharmacother. 4, 230–236 (2018).
Lachaine, J., Beauchemin, C. & Ramos, E. Use, tolerability and compliance of spironolactone in the treatment of heart failure. BMC Clin. Pharmacol. 11, 4 (2011).
Gardner, T. J., Miller, M. A., O’Gara, P. T. & Gelijns, A. C. Building an infrastructure for clinical trials in cardiac surgery. J. Thorac. Cardiovasc. Surg. 142, 265–266 (2011).
Moore, T. J., Heyward, J., Anderson, G. & Alexander, G. C. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015-2017: a cross-sectional study. BMJ Open 10, e038863 (2020).
Eisenstein, E. L. et al. Reducing the costs of phase III cardiovascular clinical trials. Am. Heart J. 149, 482–488 (2005).
Fröbert, O. et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 369, 1587–1597 (2013).
Neal, B. et al. Effect of salt substitution on cardiovascular events and death. N. Engl. J. Med. 385, 1067–1077 (2021).
Bikdeli, B. et al. Two decades of cardiovascular trials with primary surrogate endpoints: 1990–2011. J. Am. Heart Assoc. 6, e005285 (2017).
Marquis-Gravel, G. et al. Technology-enabled clinical trials. Circulation 140, 1426–1436 (2019).
Inan, O. T. et al. Digitizing clinical trials. NPJ Digit. Med. 3, 101 (2020).
Mori, M. et al. The promise of big data and digital solutions in building a cardiovascular learning system: opportunities and barriers. Methodist Debakey Cardiovasc. J. 16, 212–219 (2020).
Wolfenden, L. et al. Designing and undertaking randomised implementation trials: guide for researchers. BMJ 372, m3721 (2021).
Curran, G. M., Bauer, M., Mittman, B., Pyne, J. M. & Stetler, C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care 50, 217–226 (2012).
Gitlin, L. N. et al. Dissemination and implementation of evidence-based dementia care using embedded pragmatic trials. J. Am. Geriatr. Soc. 68, S28–S36 (2020).
Hernán, M. A., Brumback, B. & Robins, J. M. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J. Am. Stat. Assoc. 96, 440–448 (2001).
Branson, M. & Whitehead, J. Estimating a treatment effect in survival studies in which patients switch treatment. Stat. Med. 21, 2449–2463 (2002).
Latimer, N. R., Abrams, K. R., Lambert, P. C., Morden, J. P. & Crowther, M. J. Assessing methods for dealing with treatment switching in clinical trials: a follow-up simulation study. Stat. Methods Med. Res. 27, 765–784 (2018).
Mark, S. D. & Robins, J. M. A method for the analysis of randomized trials with compliance information: an application to the Multiple Risk Factor Intervention Trial. Control. Clin. Trials 14, 79–97 (1993).
Cook, A. J., Delong, E., Murray, D. M., Vollmer, W. M. & Heagerty, P. J. Statistical lessons learned for designing cluster randomized pragmatic clinical trials from the NIH Health Care Systems Collaboratory Biostatistics and Design Core. Clin. Trials 13, 504–512 (2016).
Sepehrvand, N. et al. Trends in the explanatory or pragmatic nature of cardiovascular clinical trials over 2 decades. JAMA Cardiol. 4, 1122–1128 (2019).
Van Spall, H. G. C. et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 321, 753–761 (2019).
Van Spall, H. G. C. et al. Knowledge to action: rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial. Am. Heart J. 199, 75–82 (2018).
Van Spall, H. G. C. et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur. J. Heart Fail. 19, 1427–1443 (2017).
Feltner, C. et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann. Intern. Med. 160, 774–784 (2014).
Spertus, J. A. et al. Novel trial design: CHIEF-HF. Circ. Heart Fail. 14, e007767 (2021).
Spertus, J. A. et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat. Med. 28, 809–813 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04564742 (2022).
Dockendorf, M. F. et al. Leveraging digital health technologies and outpatient sampling in clinical drug development: a phase I exploratory study. Clin. Pharmacol. Ther. 105, 168–176 (2019).
Mentz, R. J. et al. Good clinical practice guidance and pragmatic clinical trials: balancing the best of both worlds. Circulation 133, 872–880 (2016).
World Health Organization. Guidelines for Good Clinical Practice (GCP) for Trials on Pharmaceutical Products http://www.femh-irb.org/content_pages/files_add/doc_arb/I01_9712011000.pdf (1995).
International Council for Harmonisation. ICH-E6 Good Clinical Practice (GCP), Explanatory Note https://database.ich.org/sites/default/files/ICH_E6-R3_GCP-Principles_Draft_2021_0419.pdf (2021).
Claerhout, B. et al. Federated electronic health records research technology to support clinical trial protocol optimization: evidence from EHR4CR and the InSite platform. J. Biomed. Inf. 90, 103090 (2019).
Hernandez, A. F., Fleurence, R. L. & Rothman, R. L. The ADAPTABLE trial and PCORnet: shining light on a new research paradigm. Ann. Intern. Med. 163, 635–636 (2015).
Miksad, R. A. & Abernethy, A. P. Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clin. Pharmacol. Ther. 103, 202–205 (2018).
Kwakkenbos, L. et al. CONSORT extension for the reporting of randomised controlled trials conducted using cohorts and routinely collected data (CONSORT-ROUTINE): checklist with explanation and elaboration. BMJ 373, n857 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04727073 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02901184 (2021).
Jones, W. S. et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N. Engl. J. Med. 384, 1981–1990 (2021).
ASCEND Study Collaborative Groupet al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379, 1529–1539 (2018).
Choudhry, N. K. et al. Full coverage for preventive medications after myocardial infarction. N. Engl. J. Med. 365, 2088–2097 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05130268 (2022).
Greene, S. J. et al. Pragmatic design of randomized clinical trials for heart failure: rationale and design of the TRANSFORM-HF Trial. JACC Heart Fail. 9, 325–335 (2021).
Ambrosy, A. P. et al. Rationale and design of the pragmatic randomized trial of icosapent ethyl for high cardiovascular risk adults (MITIGATE). Am. Heart J. 235, 54–64 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04564742 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04509674 (2022).
Author information
Authors and Affiliations
Contributions
M.S.U., Z.A.A., R.J.M. and M.S.K. researched data for the article. H.G.C.V., S.J.G., A.P., D.K.M. and S.K.J. contributed substantially to discussion of the content. M.S.U., D.K.M., R.J.M., G.C.F., J.A.S., S.D.A., J.B. and M.S.K. wrote the article. H.G.C.V., S.J.G., A.P., D.K.M., Z.A.A., G.C.F., J.A.S., S.D.A., J.B., S.K.J. and M.S.K. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.G.C.V. is funded by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. S.J.G. has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics and Sanofi; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, PharmaIN, Roche Diagnostics, Sanofi, Tricog Health, Urovant Pharmaceuticals and Vifor. D.K.M. reports honoraria for clinical trial leadership from AbbVie, Akebia, Arena, AstraZeneca, Boehringer Ingelheim, CSL Behring, Dynavax, Eidos, Esperion, Lexicon, Lilly USA, Merck & Co, Novo Nordisk, Otsuka, Pfizer and Sanofi, and honoraria for consultancy from Afimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lilly USA, Merck & Co, Metavant, Novo Nordisk and Sanofi. Z.A.A. reports institutional research grants to Columbia University from Abbott and Cardiovascular Systems; and is a consultant for Abbott, Abiomed, AstraZeneca and Shockwave. R.J.M. reports receiving personal fees from Amgen, Bayer, Boehringer Ingelheim, Merck & Co and Novartis International; and receiving research support and honoraria from Abbott Laboratories, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly & Co, Boston Scientific Corporation, Cytokinetics, FAST BioMedical, Gilead Sciences, Innolife, Medtronic, Merck & Co, Novartis International, Relypsa, Respicardia, Windtree Therapeutics and ZOLL Medical Corporation. G.C.F. reports research support from the National Institutes of Health and consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck and Novartis. J.A.S. is a consultant for Bayer, Janssen, Merck, Myokardia, Novartis, Terumo and United Healthcare; receives grant support from Janssen and Myokadia; and holds the copyright to the Peripheral Artery Questionnaire, Kansas City Cardiomyopathy Questionnaires and the Seattle Angina Questionnaire; and serves on the Board of Blue Cross/Blue Shield of Kansas City. S.D.A. declares grants or personal fees from Abbott Vascular, Actimed, Amgen, AstraZeneca, Bayer, Bioventrix, Boehringer Ingelheim, Brahms, Cardiac Dimensions, Cordio, Janssen, Occlutech, Respicardia, Servier, Vifor Int. and V-Wave. J.B. has served as a consultant for Abbott, Adrenomed, Arena Pharma, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Cardior, CVRx, Eli Lilly, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Sequana Medical, V-Wave Limited and Vifor. S.K.J. has received institutional research/grant support from AstraZeneca, Bayer, Janssen and Novartis. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Naveed Sattar, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usman, M.S., Van Spall, H.G.C., Greene, S.J. et al. The need for increased pragmatism in cardiovascular clinical trials. Nat Rev Cardiol 19, 737–750 (2022). https://doi.org/10.1038/s41569-022-00705-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-022-00705-w
This article is cited by
-
Poor reporting quality and high proportion of missing data in economic evaluations alongside pragmatic trials: a cross-sectional survey
BMC Medical Research Methodology (2025)
-
Large simple randomized controlled trials—from drugs to medical devices: lessons from recent experience
Trials (2025)
-
Digital Therapeutics in Cardiovascular Healthcare: A Narrative Review
Current Cardiology Reports (2025)
-
How to make cardiology clinical trials more inclusive
Nature Medicine (2024)
-
Translational Science in Vascular Aging: From Bench to Bedside—Insights from a VascAgeNet Roundtable
Artery Research (2024)