Abstract
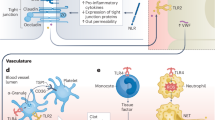
Systemic inflammation has been suggested to have a pivotal role in atherothrombosis, but the factors that trigger systemic inflammation have not been fully elucidated. Lipopolysaccharide (LPS) is a component of the membrane of Gram-negative bacteria present in the gut that can translocate into the systemic circulation, causing non-septic, low-grade endotoxaemia. Gut dysbiosis is a major determinant of low-grade endotoxaemia via dysfunction of the intestinal barrier scaffold, which is a prerequisite for LPS translocation into the systemic circulation. Experimental studies have demonstrated that LPS is present in atherosclerotic arteries but not in normal arteries. In atherosclerotic plaques, LPS promotes a pro-inflammatory status that can lead to plaque instability and thrombus formation. Low-grade endotoxaemia affects several cell types, including leukocytes, platelets and endothelial cells, leading to inflammation and clot formation. Low-grade endotoxaemia has been described in patients at risk of or with overt cardiovascular disease, in whom low-grade endotoxaemia was associated with atherosclerotic burden and its clinical sequelae. In this Review, we describe the mechanisms favouring the development of low-grade endotoxaemia, focusing on gut dysbiosis and changes in gut permeability; the plausible biological mechanisms linking low-grade endotoxaemia and atherothrombosis; the clinical studies suggesting that low-grade endotoxaemia is a risk factor for cardiovascular events; and the potential therapeutic tools to improve gut permeability and eventually eliminate low-grade endotoxaemia.
Key points
-
Gut permeability can be altered by gut microbiota dysbiosis, favouring lipopolysaccharide (LPS) translocation into the systemic circulation, with ensuing development of low-grade endotoxaemia.
-
Low-grade endotoxaemia induces an inflammatory state in the arterial wall that ultimately leads to initiation and progression of atherosclerosis.
-
Low-grade endotoxaemia has effects on several cell types, such as leukocytes, platelets and endothelial cells, shifting them to a procoagulant phenotype that contributes to thrombosis.
-
Gut permeability-derived low-grade endotoxaemia might contribute to atherosclerosis and be associated with cardiovascular events in patients at risk of or with overt cardiovascular disease.
-
Modulation of gut permeability-derived low-grade endotoxaemia is a potential tool to counteract inflammation-related atherothrombosis and its clinical sequelae.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ross, R. Inflammation or atherogenesis. N. Engl. J. Med. 340, 115–126 (1999).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Shibata, N. & Glass, C. K. Regulation of macrophage function in inflammation and atherosclerosis. J. Lipid Res. 50, S277–S281 (2009).
Violi, F., Loffredo, L., Carnevale, R., Pignatelli, P. & Pastori, D. Atherothrombosis and oxidative stress: mechanisms and management in elderly. Antioxid. Redox Signal. 27, 1083–1124 (2017).
Tabas, I. 2016 Russell Ross Memorial Lecture in Vascular Biology: Molecular–Cellular Mechanisms in the Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 183–189 (2017).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The human microbiota in health and disease. Engineering 3, 71–82 (2017).
O’Toole, P. W. & Jeffery, I. B. Gut microbiota and aging. Science 350, 1214–1215 (2015).
Ascher, S. & Reinhardt, C. The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur. J. Immunol. 48, 564–575 (2018).
Caesar, R., Fåk, F. & Bäckhed, F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism: review. J. Intern. Med. 268, 320–328 (2010).
Moreira, A. P. B., Texeira, T. F. S., Ferreira, A. B., Do Carmo Gouveia Peluzio, M. & De Cássia Gonçalves Alfenas, R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 108, 801–809 (2012).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
den Dekker, W. K., Cheng, C., Pasterkamp, G. & Duckers, H. J. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis 209, 314–320 (2010).
Carnevale, R. et al. Low-grade endotoxaemia enhances artery thrombus growth via toll-like receptor 4: implication for myocardial infarction. Eur. Heart J. 41, 3156–3165 (2020).
Koupenova, M., Clancy, L., Corkrey, H. A. & Freedman, J. E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 122, 337–351 (2018).
Koupenova, M. & Freedman, J. E. Platelets and COVID-19: inflammation, hyperactivation and additional questions. Circ. Res. 127, 1419–1421 (2020).
Wiedermann, C. J. et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J. Am. Coll. Cardiol. 34, 1975–1981 (1999).
Oliva, A. et al. Low-grade endotoxemia and thrombosis in COVID-19. Clin. Transl. Gastroenterol. 12, e00348 (2021).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Guerville, M. et al. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am. J. Physiol. Endocrinol. Metab. 313, E107–E120 (2017).
Carnevale, R. et al. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: effect of extra-virgin olive oil. Eur. J. Nutr. 58, 843–851 (2019).
Ghoshal, S., Witta, J., Zhong, J., de Villiers, W. & Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50, 90–97 (2009).
Manco, M., Putignani, L. & Bottazzo, G. F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 31, 817–844 (2010).
Stoll, L. L., Denning, G. M. & Weintraub, N. L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 2227–2236 (2004).
Levels, J. H. M. et al. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect. Immun. 73, 2321–2326 (2005).
Flegel, W. A., Wolpl, A., Mannel, D. N. & Northoff, H. Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect. Immun. 57, 2237–2245 (1989).
Levine, D. M., Parker, T. S., Donnelly, T. M., Walsh, A. & Rubin, A. L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl Acad. Sci. USA 90, 12040–12044 (1993).
Parker, T. S. et al. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect. Immun. 63, 253–258 (1995).
Guerville, M. & Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Liver Physiol. 311, G1–G15 (2016).
Carpino, G. et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 72, 470–485 (2020).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Park, B. S. & Lee, J. O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 45, e66 (2013).
Pålsson-McDermott, E. M. & O’Neill, L. A. J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113, 153–162 (2004).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Sig. Transduct. Target. Ther. 2, 17023 (2017).
Buckley, A. & Turner, J. R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 10, a029314 (2018).
Johansson, M. E. V., Sjövall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Sturgeon, C. & Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 4, e1251384 (2016).
Farquhar, M. G. & Palade, G. E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Ghosh, S., Whitley, C. S., Haribabu, B. & Jala, V. R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol. Gastroenterol. Hepatol. 11, 1463–1482 (2021).
Matsuda, M., Kubo, A., Furuse, M. & Tsukita, S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 117, 1247–1257 (2004).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Mouries, J. et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228 (2019).
Paradis, T., Bègue, H., Basmaciyan, L., Dalle, F. & Bon, F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int. J. Mol. Sci. 22, 2506 (2021).
Cohen, L. J. et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549, 48–53 (2017).
Mydel, P. et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during porphyromonas gingivalis infection. PLoS Pathog. 2, e76 (2006).
Hiippala, K. et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10, 988 (2018).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Nagpal, R. et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr. Healthy Aging 4, 267–285 (2018).
Odamaki, T. et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16, 90 (2016).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e4 (2017).
Fort, M. M. et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174, 6416–6423 (2005).
Wang, W., Xia, T. & Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 64, 423–431 (2015).
Ungaro, R. et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1167–G1179 (2009).
Peterson, C. Y. et al. Toll-like receptor-4 mediates intestinal barrier breakdown after thermal injury. Surg. Infect. 11, 137–144 (2010).
Bentala, H. et al. Removal of phosphate from lipid a as a strategy to detoxify lipopolysaccharide. Shock 18, 561–566 (2002).
Ghosh, S. S. et al. Over-expression of intestinal alkaline phosphatase attenuates atherosclerosis. Circ. Res. 128, 1646–1659 (2021).
Han, Y. H. et al. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 373, eabe6729 (2021).
Kriegel, M. A. et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 108, 11548–11553 (2011).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Wood Heickman, L. K., DeBoer, M. D. & Fasano, A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab. Res. Rev. 36, e3309 (2020).
Fasano, A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 10, 1096–1100 (2012).
Li, X. & Atkinson, M. A. The role for gut permeability in the pathogenesis of type 1 diabetes–a solid or leaky concept? Pediatr. Diabetes 16, 485–492 (2015).
Jayashree, B. et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 388, 203–210 (2014).
Aasbrenn, M., Lydersen, S. & Farup, P. G. Changes in serum zonulin in individuals with morbid obesity after weight-loss interventions: a prospective cohort study. BMC Endocr. Disord. 20, 108 (2020).
Cangemi, R. et al. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J. Infect. 73, 107–114 (2016).
Hanada, S., Pirzadeh, M., Carver, K. Y. & Deng, J. C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front. Immunol. 9, 2640 (2018).
Zhou, X. et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 6, 66 (2018).
Smith, S. M., Eng, R. H. K., Campos, J. M. & Chmel, H. D-Lactic acid measurements in the diagnosis of bacterial infections. J. Clin. Microbiol. 27, 385–388 (1989).
Sun, X. Q. et al. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J. Gastroenterol. 7, 555–558 (2001).
Fukui, H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J. Hepatol. 7, 425–442 (2015).
Sequeira, I. R., Lentle, R. G., Kruger, M. C. & Hurst, R. D. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PLoS ONE 9, e99256 (2014).
Mundi, S. et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors–a review. Cardiovasc. Res. 114, 35–52 (2018).
Wu, M. Y., Li, C. J., Hou, M. F. & Chu, P. Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 18, 2034 (2017).
Iuliano, L., Mauriello, A., Sbarigia, E., Spagnoli, L. G. & Violi, F. Radiolabeled native low-density lipoprotein injected into patients with carotid stenosis accumulates in macrophages of atherosclerotic plaque. Circulation 101, 1249–1254 (2000).
Jonsson, A. L. & Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14, 79–87 (2017).
Karlsson, F. H. et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3, 1245 (2012).
Emoto, T. et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessel. 32, 39–46 (2017).
Kiouptsi, K. et al. The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice. MBio 10, e02298-19 (2019).
Violi, F., Carnevale, R., Loffredo, L., Pignatelli, P. & Gallin, J. I. NADPH oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. Arterioscler. Thromb. Vasc. Biol. 37, 218–225 (2017).
Nocella, C. et al. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb. Haemost. 117, 1558–1570 (2017).
Carnevale, R. et al. LDL are oxidatively modified by platelets via GP91(phox) and accumulate in human monocytes. FASEB J. 21, 927–934 (2007).
Carnevale, R. et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 237, 108–116 (2014).
Carnevale, R. et al. Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr. Metab. Cardiovasc. Dis. 27, 890–895 (2017).
Carnevale, R. et al. Localization of lipopolysaccharide from Escherichia coli into human atherosclerotic plaque. Sci. Rep. 8, 3598 (2018).
Levels, J. H. M., Abraham, P. R., Van Barreveld, E. P., Meijers, J. C. M. & Van Deventer, S. J. H. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect. Immun. 71, 3280–3284 (2003).
Hersoug, L. G., Møller, P. & Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 31, 153–163 (2018).
Rehues, P. et al. Characterization of the LPS and 3OHFA contents in the lipoprotein fractions and lipoprotein particles of healthy men. Biomolecules 12, 47 (2021).
Memon, R. A. et al. Infection and inflammation induce LDL oxidation in vivo. Arterioscler. Thromb. Vasc. Biol. 20, 1536–1542 (2000).
Fuijkschot, W. W. et al. LPS-induced systemic inflammation does not alter atherosclerotic plaque area or inflammation in ApoE3*Leiden mice in the early phase up to 15 days. Shock 50, 360–365 (2018).
Lehr, H. A. et al. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation 104, 914–920 (2001).
Ostos, M. A., Recalde, D., Zakin, M. M. & Scott-Algara, D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 519, 23–29 (2002).
Rice, J. B. et al. Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler. Thromb. Vasc. Biol. 23, 1576–1582 (2003).
Violi, F., Calvieri, C., Ferro, D. & Pignatelli, P. Statins as antithrombotic drugs. Circulation 127, 251–257 (2013).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Vink, A. et al. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation 106, 1985–1990 (2002).
Ding, Y. et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 1596–1604 (2012).
Koupenova, M., Livada, A. C. & Morrell, C. N. Platelet and megakaryocyte roles in innate and adaptive immunity. Circ. Res. 130, 288–308 (2022).
Jaw, J. E. et al. Lung exposure to lipopolysaccharide causes atherosclerotic plaque destabilisation. Eur. Respir. J. 48, 205–215 (2016).
Schumski, A. et al. Endotoxinemia accelerates atherosclerosis through electrostatic charge-mediated monocyte adhesion. Circulation 143, 254–266 (2021).
Mawhin, M.-A. et al. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc. Res. 114, 1656–1666 (2018).
Zhu, W. et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124 (2016).
Violi, F. et al. Bleeding time in patients with cirrhosis: relation with degree of liver failure and clotting abnormalities. J. Hepatol. 20, 531–536 (1994).
Violi, F. et al. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology 109, 531–539 (1995).
Fimognari, F. L. & Violi, F. Portal vein thrombosis in liver cirrhosis. Intern. Emerg. Med. 3, 213–218 (2008).
Saliola, M. et al. Enhanced expression of monocyte tissue factor in patients with liver cirrhosis. Gut 43, 428–432 (1998).
Camerer, E., Huang, W. & Coughlin, S. R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl Acad. Sci. USA 97, 5255–5260 (2000).
Ferro, D. et al. Vitamin E reduces monocyte tissue factor expression in cirrhotic patients. Blood 93, 2945–2950 (1999).
Zhang, W. J., Wojta, J. & Binder, B. R. Notoginsenoside R1 counteracts endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo. Arterioscler. Thromb. Vasc. Biol. 17, 465–474 (1997).
Ruan, Q., Deng, Z. & Song, J. Ligustrazini inhibits endotoxin induced PAI-1 expression in human umbilical vein endothelial cells. J. Tongji Med. Univ. 21, 6–7 (2001).
Ren, M. et al. Endothelial cells but not platelets are the major source of Toll-like receptor 4 in the arterial thrombosis and tissue factor expression in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R901–R907 (2014).
Carnevale, R. et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J. Hepatol. 67, 950–956 (2017).
Sambrano, G. R. et al. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 275, 6819–6823 (2000).
Nadir, Y. et al. Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII. Haematologica 95, 1927–1934 (2010).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Yao, W. et al. ONO-5046 suppresses reactive oxidative species-associated formation of neutrophil extracellular traps. Life Sci. 210, 243–250 (2018).
Khan, M. A. et al. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci. Rep. 7, 3409 (2017).
Barillà, F. et al. Toll-like receptor 4 activation in platelets from myocardial infarction patients. Thromb. Res. 209, 33–40 (2022).
Jäckel, S. et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood 130, 542–553 (2017).
Asada, M. et al. Serum lipopolysaccharide-binding protein levels and the incidence of cardiovascular disease in a general Japanese population: the Hisayama study. J. Am. Heart Assoc. 8, e013628 (2019).
Leskelä, J. et al. Genetic profile of endotoxemia reveals an association with thromboembolism and stroke. J. Am. Heart Assoc. 10, e022482 (2021).
Pussinen, P. J. et al. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler. Thromb. Vasc. Biol. 27, 1433–1439 (2007).
Gomes, J. M. G., de Costa, J. A. & de Alfenas, R. C. G. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism 68, 133–144 (2017).
Simonsen, J. R. et al. The association between bacterial infections and the risk of coronary heart disease in type 1 diabetes. J. Intern. Med. 288, 711–724 (2020).
Pastori, D. et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to Mediterranean diet. J. Am. Heart Assoc. 6, e005784 (2017).
Zhang, Y. et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 118, 785–797 (2021).
Cangemi, R. et al. Comparison of thrombotic events and mortality in patients with community-acquired pneumonia and COVID-19: a multicenter observational study. Thromb. Haemost. 122, 257–266 (2021).
Malehmir, M. et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 25, 641–655 (2019).
Baratta, F. et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin. Gastroenterol. Hepatol. 18, 2324–2331.e4 (2020).
Amar, J. Microbiota-host crosstalk: a bridge between cardiovascular risk factors, diet, and cardiovascular disease. Am. J. Hypertens. 31, 941–944 (2018).
De Filippis, F. et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821 (2016).
Bartimoccia, S. et al. Extra virgin olive oil reduces gut permeability and metabolic endotoxemia in diabetic patients. Nutrients 14, 2153 (2022).
Guevara-Cruz, M. et al. Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. J. Am. Heart Assoc. 8, e012401 (2019).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Rault-Nania, M. H. et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br. J. Nutr. 96, 840–844 (2006).
Dourado, E., Ferro, M., Guerreiro, C. S. & Fonseca, J. E. Diet as a modulator of intestinal microbiota in rheumatoid arthritis. Nutrients 12, 3504 (2020).
Knudsen, K. E. B. et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10, 1499 (2018).
Rosenson, R. S. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis 173, 1–12 (2004).
Zhang, S. et al. Atorvastatin attenuates cold-induced hypertension by preventing gut barrier injury. J. Cardiovasc. Pharmacol. 74, 143–151 (2019).
Vieira-Silva, S. et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315 (2020).
Fukui, H. Leaky gut and gut-liver axis in liver cirrhosis: clinical studies update. Gut Liver 15, 666–676 (2021).
Munford, R. S. Endotoxemia–menace, marker, or mistake? J. Leukoc. Biol. 100, 687–698 (2016).
Rinninella, E. et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7, 14 (2019).
Thursby, E. & Juge, N. Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836 (2017).
Berg, R. D. The indigenous gastrointestinal microflora. Trends Microbiol. 4, 430–435 (1996).
Cani, P. D. Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725 (2018).
Dieterich, W., Schink, M. & Zopf, Y. Microbiota in the gastrointestinal tract. Med. Sci. 6, 116 (2018).
Shin, N. R., Whon, T. W. & Bae, J. W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503 (2015).
Stojanov, S., Berlec, A. & Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8, 1715 (2020).
Deo, P. N. & Deshmukh, R. Oral microbiome: unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 23, 122–128 (2019).
Chen, C. et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 12, 1210–1224 (2018).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018).
Del Giudice, C. et al. Infective endocarditis: a focus on oral microbiota. Microorganisms 9, 1218 (2021).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl Acad. Sci. USA 108, 4592–4598 (2011).
Bensel, T. et al. Periodontal pockets as potential sources of cystic fibrosis lung infection. J. Cyst. Fibros. 9, S38 (2010).
Tan, L., Wang, H., Li, C. & Pan, Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J. Periodontal Res. 49, 760–769 (2014).
Prescott, S. L. et al. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 10, 29 (2017).
Sanford, J. A. & Gallo, R. L. Functions of the skin microbiota in health and disease. Semin. Immunol. 25, 370–377 (2013).
Morand, A. et al. Human bacterial repertoire of the urinary tract: a potential paradigm shift. J. Clin. Microbiol. 57, e00675-18 (2019).
Nielubowicz, G. R. & Mobley, H. L. T. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 7, 430–441 (2010).
Ulett, G. C. et al. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr. Opin. Microbiol. 16, 100–107 (2013).
Kamisoglu, K. et al. Temporal metabolic profiling of plasma during endotoxemia in humans. Shock 40, 519–526 (2013).
Määttä, A. M. et al. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. 27, 3–14 (2021).
Fong, Y. et al. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J. Clin. Invest. 85, 1896–1904 (1990).
Bang, F. B. A bacterial disease of Limulus polyphemus. Bull. Johns. Hopkins Hosp. 98, 325–351 (1956).
Levin, J. & Bang, F. B. The role of endotoxin in the extracellular coagulation of limulus blood. Bull. Johns. Hopkins Hosp. 115, 265–274 (1964).
Sondhi, P., Maruf, M. H. U. & Stine, K. J. Nanomaterials for biosensing lipopolysaccharide. Biosensors 10, 2 (2020).
Grallert, H., Leopoldseder, S., Schuett, M., Kurze, P. & Buchberger, B. EndoLISA®: a novel and reliable method for endotoxin detection. Nat. Methods 8, iii–v (2011).
Maitra, S. K., Schotz, M. C., Yoshikawa, T. T. & Guze, L. B. Determination of lipid A and endotoxin in serum by mass spectroscopy. Proc. Natl Acad. Sci. USA 75, 3993–3997 (1978).
De Barros, J. P. P. et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the Limulus amebocyte lysate assay. J. Lipid Res. 56, 1363–1369 (2015).
Ding, X., Su, W. & Ding, X. Methods of endotoxin detection. J. Lab. Autom. 20, 354–364 (2015).
Xie, P. et al. Highly sensitive detection of lipopolysaccharides using an aptasensor based on hybridization chain reaction. Sci. Rep. 6, 29524 (2016).
Author information
Authors and Affiliations
Contributions
F.V. developed the concept and design of the review and wrote the manuscript. All the authors contributed to the discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Christoph Reinhardt; Christoph Thiemermann, who co-reviewed with Shireen Mohammad; Pirkko Pussinen; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Gut dysbiosis
-
Any change in the number or diversity of resident commensal gut microbiota relative to the community present in healthy individuals.
- Trimethylamine-N-oxide
-
(TMAO). A small organic compound formed from trimethylamine, which is generated by metabolism of dietary choline and phosphatidylcholine by flavin monooxygenases from gut microbiota.
- Endotoxaemia
-
The presence in the blood of endotoxins, such as lipopolysaccharide (LPS), which is a component of the outer membrane of Gram-negative bacteria.
- Non-alcoholic fatty liver disease
-
(NAFLD). A condition caused by ectopic fat accumulation in the liver in the absence of excessive alcohol intake.
- Non-alcoholic steatohepatitis
-
(NASH). A severe form of non-alcoholic fatty liver disease that is characterized by liver inflammation and that can progress to cirrhosis.
- Intestinal adhesion proteins
-
The scaffold of the epithelial cell barrier that protects from the translocation of microorganisms or microbial products into the systemic circulation.
- Increased gut permeability
-
Impaired function of epithelial cell adhesion proteins and loss of epithelial cell barrier integrity, with translocation of microorganisms and toxins into the bloodstream.
- NADPH oxidase
-
A ubiquitous, multisubunit cellular enzyme that is a major producer of reactive oxygen species.
- Neutrophil extracellular traps
-
(NETs). Extracellular net-like structures composed of DNA, histones and cytoplasmic granule proteins released by neutrophils after activation.
- Prebiotics
-
Non-digestible food ingredients (fibres) that promote a shift to a healthier gut microbiota profile.
- Probiotics
-
Live microorganisms that confer a health benefit in the host when administered in adequate amounts.
Rights and permissions
About this article
Cite this article
Violi, F., Cammisotto, V., Bartimoccia, S. et al. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol 20, 24–37 (2023). https://doi.org/10.1038/s41569-022-00737-2
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41569-022-00737-2
This article is cited by
-
Amelioration of gut dysbiosis-induced cognitive deterioration by repeated administration of human clostridium butyricum: targeting intestinal and blood–brain barrier
Future Journal of Pharmaceutical Sciences (2025)
-
Systemic inflammation as a central player in the initiation and development of Alzheimer’s disease
Immunity & Ageing (2025)
-
The role of the gut microbiota and its metabolites: a new predictor in diabetes and its complications
European Journal of Medical Research (2025)
-
Pathogenicity of Arcobacter cryaerophilus in two human intestinal cell lines
Gut Pathogens (2025)
-
Inflammation and resolution in obesity
Nature Reviews Endocrinology (2025)