Abstract
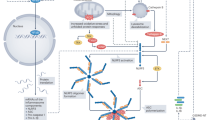
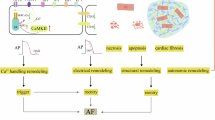
Inflammation has been implicated in atrial fibrillation (AF), a very common and clinically significant cardiac rhythm disturbance, but its precise role remains poorly understood. Work performed over the past 5 years suggests that atrial cardiomyocytes have inflammatory signalling machinery — in particular, components of the NLRP3 (NACHT-, LRR- and pyrin domain-containing 3) inflammasome — that is activated in animal models and patients with AF. Furthermore, work in animal models suggests that NLRP3 inflammasome activation in atrial cardiomyocytes might be a sufficient and necessary condition for AF occurrence. In this Review, we evaluate the evidence for the role and pathophysiological significance of cardiomyocyte NLRP3 signalling in AF. We first summarize the evidence for a role of inflammation in AF and review the biochemical properties of the NLRP3 inflammasome, as defined primarily in studies of classic inflammation. We then briefly consider the broader evidence for a role of inflammatory signalling in heart disease, particularly conditions that predispose individuals to develop AF. We provide a detailed discussion of the available information about atrial cardiomyocyte NLRP3 inflammasome signalling in AF and related conditions and evaluate the possibility that similar signalling might be important in non-myocyte cardiac cells. We then review the evidence on the role of active resolution of inflammation and its potential importance in suppressing AF-related inflammatory signalling. Finally, we consider the therapeutic potential and broader implications of this new knowledge and highlight crucial questions to be addressed in future research.
Key points
-
Atrial fibrillation is the most common cardiac arrhythmia, and new insights into the pathophysiological mechanisms are required to improve the available therapeutic options.
-
Although inflammation has been thought to have a role in atrial fibrillation for ≥20 years, the precise nature of the association has proved elusive.
-
Work over the past 5 years has revealed a key role for inflammatory signalling in cardiomyocytes in atrial fibrillation development, revealing the importance of non-leukocyte cardiac cells in mediating disease-promoting cardiac inflammation.
-
The NLRP3 inflammasome in particular has been implicated in cardiomyocyte-mediated inflammatory signalling in atrial fibrillation.
-
The active resolution of inflammation is thought to be mediated by endogenous bioactive agents, which can be administered as pharmaceutical products to attenuate the development of chronic conditions such as atrial fibrillation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Heijman, J., Guichard, J. B., Dobrev, D. & Nattel, S. Translational challenges in atrial fibrillation. Circ. Res. 122, 752–773 (2018).
Chung, M. K. et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104, 2886–2891 (2001).
Harada, M., Van Wagoner, D. R. & Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 79, 495–502 (2015).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Zhao, C. & Zhao, W. NLRP3 inflammasome – a key player in antiviral responses. Front. Immunol. 11, 211 (2020).
Lazzerini, P. E., Capecchi, P. L. & Laghi-Pasini, F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur. Heart J. 38, 1717–1727 (2017).
Ahlehoff, O. et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish nationwide cohort study. Eur. Heart J. 33, 2054–2064 (2012).
Kristensen, S. L. et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace 16, 477–484 (2014).
Koyfman, L. et al. Epidemiology of new-onset paroxysmal atrial fibrillation in the general intensive care unit population and after discharge from ICU. A retrospective epidemiological study. Anaesthesiol. Intensive Ther. 47, 309–314 (2015).
Walkey, A. J., Wiener, R. S., Ghobrial, J. M., Curtis, L. H. & Benjamin, E. J. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 306, 2248–2254 (2011).
Walkey, A. J. et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am. Heart J. 165, 949–955.e3 (2013).
Kuipers, S., Klein Klouwenberg, P. M. & Cremer, O. L. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit. Care 18, 688 (2014).
Walkey, A. J., Hammill, B. G., Curtis, L. H. & Benjamin, E. J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 146, 1187–1195 (2014).
Klein Klouwenberg, P. M. et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. a cohort study. Am. J. Respir. Crit. Care Med. 195, 205–211 (2017).
Cheng, C. A. et al. New-onset atrial fibrillation-related ischemic stroke occurring after hospital discharge in septicemia survivors. QJM 110, 453–457 (2017).
Bosch, N. A. et al. New-onset atrial fibrillation as a sepsis-defining organ failure. Ann. Am. Thorac. Soc. 16, 1332–1334 (2019).
Fernando, S. M. et al. New-onset atrial fibrillation and associated outcomes and resource use among critically ill adults – a multicenter retrospective cohort study. Crit. Care 24, 15 (2020).
Ko, D. et al. Secondary precipitants of atrial fibrillation and anticoagulation therapy. J. Am. Heart Assoc. 10, e021746 (2021).
Long, Y. et al. Early coagulation disorder is associated with an increased risk of atrial fibrillation in septic patients. Front. Cardiovasc. Med. 8, 724942 (2021).
Spodick, D. H. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 235, 39–41 (1976).
Nagahama, Y. et al. The role of infarction-associated pericarditis on the occurrence of atrial fibrillation. Eur. Heart J. 19, 287–292 (1998).
Ristić, A. D. et al. Arrhythmias in acute pericarditis. An endomyocardial biopsy study. Herz 25, 729–733 (2000).
Imazio, M. et al. Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart 101, 1463–1467 (2015).
Adegbala, O. et al. Predictors, burden, and the impact of arrhythmia on patients admitted for acute myocarditis. Am. J. Cardiol. 123, 139–144 (2019).
Frustaci, A. et al. Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest 100, 303–306 (1991).
Frustaci, A. et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 96, 1180–1184 (1997).
Fuenmayor, A. J. et al. Results of electrophysiologic studies in patients with acute chagasic myocarditis. Clin. Cardiol. 20, 1021–1024 (1997).
Morgera, T. et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am. Heart J. 124, 455–467 (1992).
Deluigi, C. C. et al. ECG findings in comparison to cardiovascular MR imaging in viral myocarditis. Int. J. Cardiol. 165, 100–106 (2013).
Coromilas, E. J. et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 14, e009458 (2021).
Gawalko, M., Kaplon-Cieslicka, A., Hohl, M., Dobrev, D. & Linz, D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int. J. Cardiol. Heart Vasc. 30, 100631 (2020).
Sun, Z. et al. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic. Res. Cardiol. 111, 63 (2016).
Fang, J. et al. Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia. Eur. J. Pharmacol. 913, 174622 (2021).
Aoki, Y. et al. Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs. Br. J. Pharmacol. 166, 390–400 (2012).
Landstrom, A. P., Dobrev, D. & Wehrens, X. H. T. Calcium signaling and cardiac arrhythmias. Circ. Res. 120, 1969–1993 (2017).
Nattel, S., Heijman, J., Zhou, L. & Dobrev, D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ. Res. 127, 51–72 (2020).
Kumagai, K., Nakashima, H. & Saku, K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc. Res. 62, 105–111 (2004).
Ryu, K. et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 293, H1231–H1241 (2007).
Huang, Z. et al. Signal transducer and activator of transcription 3/microRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat sterile pericarditis model. Circ. Arrhythm. Electrophysiol. https://doi.org/10.1161/CIRCEP.115.003396 (2016).
Wu, Q. et al. Colchicine prevents atrial fibrillation promotion by inhibiting IL-1β-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model. Biomed. Pharmacother. 129, 110384 (2020).
Zhang, Y. et al. Role of inflammation in the initiation and maintenance of atrial fibrillation and the protective effect of atorvastatin in a goat model of aseptic pericarditis. Mol. Med. Rep. 11, 2615–2623 (2015).
Zhang, Z. et al. n-3 polyunsaturated fatty acids prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Int. J. Cardiol. 153, 14–20 (2011).
Chang, C. J. et al. Histone deacetylase inhibition attenuates atrial arrhythmogenesis in sterile pericarditis. Transl. Res. 200, 54–64 (2018).
Liao, J. et al. TRPV4 blockade suppresses atrial fibrillation in sterile pericarditis rats. JCI Insight https://doi.org/10.1172/jci.insight.137528 (2020).
Liao, J. et al. Interleukin-6-mediated-Ca2+ handling abnormalities contributes to atrial fibrillation in sterile pericarditis rats. Front. Immunol. 12, 758157 (2021).
Lin, F. J. et al. Toll-like receptor 4 activation modulates pericardium-myocardium interactions in lipopolysaccharide-induced atrial arrhythmogenesis. Europace 23, 1837–1846 (2021).
Schwartzman, D. et al. A plasma-based, amiodarone-impregnated material decreases susceptibility to atrial fibrillation in a post-cardiac surgery model. Innovations 11, 59–63 (2016). discussion 63.
Fu, X. X. et al. Interleukin-17A contributes to the development of post-operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int. J. Mol. Med. 36, 83–92 (2015).
Tubeeckx, M. R. L. et al. Sterile pericarditis in Aachener minipigs as a model for atrial myopathy and atrial fibrillation. J. Vis. Exp. https://doi.org/10.3791/63094 (2021).
Hoyano, M. et al. Inducibility of atrial fibrillation depends not on inflammation but on atrial structural remodeling in rat experimental autoimmune myocarditis. Cardiovasc. Pathol. 19, e149–e157 (2010).
Turner, N. A. et al. Interleukin-1α stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 297, H1117–H1127 (2009).
Wang, Y. et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin. Transl. Med. 10, 91–106 (2020).
Liu, L. et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell Mol. Med. 19, 2728–2740 (2015).
Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2022).
Scott, L. Jr., Li, N. & Dobrev, D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 287, 195–200 (2019).
Latz, E. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22, 28–33 (2010).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Groslambert, M. & Py, B. F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 11, 359–374 (2018).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 49, 331–338 (2013).
Bryan, N. B., Dorfleutner, A., Rojanasakul, Y. & Stehlik, C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182, 3173–3182 (2009).
Baroja-Mazo, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 (2014).
Dick, M. S., Sborgi, L., Ruhl, S., Hiller, S. & Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 7, 11929 (2016).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Suetomi, T. et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138, 2530–2544 (2018).
Fender, A. C. et al. Thrombin receptor PAR4 drives canonical NLRP3 inflammasome signaling in the heart. Basic. Res. Cardiol. 115, 10 (2020).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Lin, K. M. et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA 111, 775–780 (2014).
Schroder, K. et al. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology 217, 1325–1329 (2012).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Gong, T., Yang, Y., Jin, T., Jiang, W. & Zhou, R. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol. 39, 393–406 (2018).
Weber, K. & Schilling, J. D. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 289, 9158–9171 (2014).
Magupalli, V. G. et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science https://doi.org/10.1126/science.aas8995 (2020).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109, 11282–11287 (2012).
Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Tseng, H. H., Vong, C. T., Kwan, Y. W., Lee, S. M. & Hoi, M. P. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 6, 35016 (2016).
Compan, V. et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500 (2012).
Horng, T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 35, 253–261 (2014).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Gaidt, M. M. & Hornung, V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 35, 2167–2169 (2016).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 12, 59–67 (2016).
Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846 (2016).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Man, S. M. & Kanneganti, T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16, 7–21 (2016).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Yang, D., He, Y., Munoz-Planillo, R., Liu, Q. & Nunez, G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932 (2015).
He, Y., Hara, H. & Nunez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Wallach, D., Kang, T. B., Dillon, C. P. & Green, D. R. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352, aaf2154 (2016).
Aziz, M., Jacob, A., Yang, W. L., Matsuda, A. & Wang, P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J. Leukoc. Biol. 93, 329–342 (2013).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Karmakar, M. et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11, 2212 (2020).
Toldo, S. & Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214 (2018).
Abbate, A. et al. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126, 1260–1280 (2020).
Silvis, M. J. M. et al. Immunomodulation of the NLRP3 inflammasome in atherosclerosis, coronary artery disease, and acute myocardial infarction. J. Cardiovasc. Transl. Res. 14, 23–34 (2021).
Mezzaroma, E., Abbate, A. & Toldo, S. The inflammasome in heart failure. Curr. Opin. Physiol. 19, 105–112 (2021).
Rajamaki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Kawaguchi, M. et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594–604 (2011).
Sandanger, O. et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 99, 164–174 (2013).
Deftereos, S. et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 132, 1395–1403 (2015).
Heijman, J. et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. 127, 1036–1055 (2020).
Byrne, N. J. et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ. Heart Fail. 13, e006277 (2020).
Mehta, S., Vijayvergiya, R. & Dhawan, V. Activation of NLRP3 inflammasome assembly is associated with smoking status of patients with coronary artery disease. Int. Immunopharmacol. 87, 106820 (2020).
Yao, C. et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 138, 2227–2242 (2018).
Xiao, H. et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur. Heart J. 39, 60–69 (2018).
Zhuang, Y. T. et al. IL-6 induced lncRNA MALAT1 enhances TNF-α expression in LPS-induced septic cardiomyocytes via activation of SAA3. Eur. Rev. Med. Pharmacol. Sci. 21, 302–309 (2017).
Chandrasekar, B., Mummidi, S., Claycomb, W. C., Mestril, R. & Nemer, M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J. Biol. Chem. 280, 4553–4567 (2005).
Aschar-Sobbi, R. et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat. Commun. 6, 6018 (2015).
Lakin, R. et al. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J. Mol. Cell Cardiol. 129, 165–173 (2019).
Scott, L. Jr et al. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc. Res. 117, 1746–1759 (2021).
Saba, S. et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-α. Am. J. Physiol. Heart Circ. Physiol. 289, H1456–H1467 (2005).
Sawaya, S. E. et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am. J. Physiol. Heart Circ. Physiol. 292, H1561–H1567 (2007).
Lee, S. H. et al. Tumor necrosis factor-α alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 80, 1806–1815 (2007).
He, G. et al. Increased M1 macrophages infiltration is associated with thrombogenesis in rheumatic mitral stenosis patients with atrial fibrillation. PLoS ONE 11, e0149910 (2016).
Lazzerini, P. E. et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J. Am. Heart Assoc. 8, e011006 (2019).
Li, N. & Brundel, B. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ. Res. 127, 73–90 (2020).
Willeford, A. et al. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight https://doi.org/10.1172/jci.insight.97054 (2018).
Ling, H. et al. Ca2+/calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ. Res. 112, 935–944 (2013).
Yao, Y. et al. Targeting CaMKII-δ9 ameliorates cardiac ischemia/reperfusion injury by inhibiting myocardial inflammation. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.121.319478 (2022).
Joiner, M. L. et al. CaMKII determines mitochondrial stress responses in heart. Nature 491, 269–273 (2012).
Dobrev, D. & Dudley, S. C. Oxidative stress: a bystander or a causal contributor to atrial remodelling and fibrillation. Cardiovasc. Res. 117, 2291–2293 (2021).
Gawalko, M. et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvab292 (2021).
Hu, Y. F., Chen, Y. J., Lin, Y. J. & Chen, S. A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 12, 230–243 (2015).
Yu, L. et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 255, 92–98 (2018).
Wu, X. et al. Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J. Cardiovasc. Transl. Res. 13, 731–740 (2020).
Hiram, R. et al. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J. Am. Coll. Cardiol. 74, 1332–1347 (2019).
Goudis, C. A. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J. Cardiol. 69, 699–705 (2017).
Hiram, R. et al. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodelling in experimental right heart disease. Cardiovasc. Res. 117, 1776–1789 (2021).
Zhang, W. et al. Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS ONE 9, e107639 (2014).
Turner, N. A. et al. Mechanism of TNFα-induced IL-1α, IL-1β and IL-6 expression in human cardiac fibroblasts: effects of statins and thiazolidinediones. Cardiovasc. Res. 76, 81–90 (2007).
van Nieuwenhoven, F. A., Hemmings, K. E., Porter, K. E. & Turner, N. A. Combined effects of interleukin-1α and transforming growth factor-β1 on modulation of human cardiac fibroblast function. Matrix Biol. 32, 399–406 (2013).
Cheng, C. et al. Mutation in NPPA causes atrial fibrillation by activating inflammation and cardiac fibrosis in a knock-in rat model. FASEB J. 33, 8878–8891 (2019).
Gawalko, M. et al. Adiposity-associated atrial fibrillation: molecular determinants, mechanisms and clinical significance. Cardiovasc Res. https://doi.org/10.1093/cvr/cvac093 (2022).
Wong, C. X. et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ. Arrhythm. Electrophysiol. https://doi.org/10.1161/CIRCEP.116.004378 (2016).
Mazurek, T. et al. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am. J. Cardiol. 113, 1505–1508 (2014).
Shaihov-Teper, O. et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation 143, 2475–2493 (2021).
Freire, M. O. & Van Dyke, T. E. Natural resolution of inflammation. Periodontol 2000 63, 149–164 (2013).
Weissmann, G., Smolen, J. E. & Korchak, H. M. Release of inflammatory mediators from stimulated neutrophils. N. Engl. J. Med. 303, 27–34 (1980).
Serhan, C. N. et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 (2007).
Houck, J. C. Chemical Messengers of the Inflammatory Process (Elsevier/North-Holland, 1979).
Samuelsson, B. From studies of biochemical mechanism to novel biological mediators: prostaglandin endoperoxides, thromboxanes, and leukotrienes. Nobel Lecture, 8 December 1982. Biosci. Rep. 3, 791–813 (1983).
Serhan, C. N. et al. The atlas of inflammation resolution (AIR). Mol. Asp. Med. 74, 100894 (2020).
Balsinde, J., Winstead, M. V. & Dennis, E. A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 531, 2–6 (2002).
Radmark, O. & Samuelsson, B. 5-Lipoxygenase: mechanisms of regulation. J. Lipid Res. 50, S40–S45 (2009).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
Lee, S. et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am. J. Respir. Crit. Care Med. 196, 713–726 (2017).
Singh, N. K. & Rao, G. N. Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 73, 28–45 (2019).
Ishihara, T., Yoshida, M. & Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 31, 559–567 (2019).
El Kebir, D., Gjorstrup, P. & Filep, J. G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA 109, 14983–14988 (2012).
Pirault, J. & Back, M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 9, 1273 (2018).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Qu, X. et al. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 228, 506–519 (2012).
Al-Shaer, A. E., Pal, A. & Shaikh, S. R. Resolvin E1-ChemR23 axis regulates the hepatic metabolic and inflammatory transcriptional landscape in obesity at the whole genome and exon level. Front. Nutr. 8, 799492 (2021).
Markworth, J. F. et al. Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell 20, e13393 (2021).
Li, G. et al. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J. Lipid Res. 58, 1080–1090 (2017).
Younes, R., LeBlanc, C. A. & Hiram, R. Evidence of failed resolution mechanisms in arrhythmogenic inflammation, fibrosis and right heart disease. Biomolecules https://doi.org/10.3390/biom12050720 (2022).
Chiurchiu, V. et al. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J. 33, 909–916 (2019).
Artiach, G. et al. Omega-3 polyunsaturated fatty acids decrease aortic valve disease through the resolvin E1 and ChemR23 axis. Circulation 142, 776–789 (2020).
Jaen, R. I. et al. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 34, 10531–10546 (2020).
Keyes, K. T. et al. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 299, H153–H164 (2010).
Kain, V. et al. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell Cardiol. 84, 24–35 (2015).
Hiram, R. Resolution-promoting autacoids demonstrate promising cardioprotective effects against heart diseases. Mol. Biol. Rep. https://doi.org/10.1007/s11033-022-07230-6 (2022).
Gilroy, D. W. et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 (1999).
Hua, K. F. et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production. J. Cell Physiol. 230, 863–874 (2015).
Chan, M. M. & Moore, A. R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 184, 6418–6426 (2010).
Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 (2001).
Patrono, C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 82, 957–964 (2016).
Hocherl, K., Endemann, D., Kammerl, M. C., Grobecker, H. F. & Kurtz, A. Cyclo-oxygenase-2 inhibition increases blood pressure in rats. Br. J. Pharmacol. 136, 1117–1126 (2002).
Qi, Z. et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J. Clin. Invest. 110, 61–69 (2002).
Izhar, M., Alausa, T., Folker, A., Hung, E. & Bakris, G. L. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and Hispanics. Hypertension 43, 573–577 (2004).
Shiroshita-Takeshita, A., Brundel, B. J., Lavoie, J. & Nattel, S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc. Res. 69, 865–875 (2006).
Nomani, H., Mohammadpour, A. H., Moallem, S. M. H. & Sahebkar, A. Anti-inflammatory drugs in the prevention of post-operative atrial fibrillation: a literature review. Inflammopharmacology 28, 111–129 (2020).
Zhou, X. et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B 9, 711–723 (2019).
Ofman, P. et al. Aspirin use and risk of atrial fibrillation in the Physicians’ Health Study. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.113.000763 (2014).
Liu, Y. et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999).
Giles, K. M. et al. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J. Immunol. 167, 976–986 (2001).
Birnbaum, Y. et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation 114, 929–935 (2006).
Lucas, C. D. et al. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 7, 857–868 (2014).
Busillo, J. M., Azzam, K. M. & Cidlowski, J. A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 286, 38703–38713 (2011).
Panoulas, V. F. et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology 47, 72–75 (2008).
Tripathi, R. C., Parapuram, S. K., Tripathi, B. J., Zhong, Y. & Chalam, K. V. Corticosteroids and glaucoma risk. Drugs Aging 15, 439–450 (1999).
Nashel, D. J. Is atherosclerosis a complication of long-term corticosteroid treatment? Am. J. Med. 80, 925–929 (1986).
Xu, Y. et al. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int. Immunopharmacol. 9, 360–365 (2009).
Perregaux, D. G. et al. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197 (2001).
Zahid, A., Li, B., Kombe, A. J. K., Jin, T. & Tao, J. Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 10, 2538 (2019).
Gao, R. F. et al. The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice. Int. Immunopharmacol. 90, 107133 (2021).
Wei, Z. et al. Loss of Camk2n1 aggravates cardiac remodeling and malignant ventricular arrhythmia after myocardial infarction in mice via NLRP3 inflammasome activation. Free Radic. Biol. Med. 167, 243–257 (2021).
Brucato, A. et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 316, 1906–1912 (2016).
Abbate, A. et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. https://doi.org/10.1093/ehjcvp/pvab075 (2021).
Van Tassell, B. W. et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004373 (2017).
Klein, A. L. et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N. Engl. J. Med. 384, 31–41 (2021).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Thul, S., Labat, C., Temmar, M., Benetos, A. & Back, M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. Eur. J. Prev. Cardiol. 24, 903–906 (2017).
Bazan, H. A. et al. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fat. Acids 125, 43–47 (2017).
Fredman, G. Can inflammation-resolution provide clues to treat patients according to their plaque phenotype. Front. Pharmacol. 10, 205 (2019).
Petri, M. H. et al. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E(-/-) mice. Br. J. Pharmacol. 174, 4043–4054 (2017).
Viola, J. R. et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 119, 1030–1038 (2016).
Zhang, J. et al. The anti-inflammatory mediator resolvin E1 protects mice against lipopolysaccharide-induced heart injury. Front. Pharmacol. 11, 203 (2020).
Halade, G. V., Kain, V., Black, L. M., Prabhu, S. D. & Ingle, K. A. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging 8, 2611–2634 (2016).
Halade, G. V. & Lee, D. H. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine 79, 103992 (2022).
El-Sharkawy, L. Y., Brough, D. & Freeman, S. Inhibiting the NLRP3 Inflammasome. Molecules https://doi.org/10.3390/molecules25235533 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04338997 (2020).
European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000489-40/GB (2020).
Van Tassell, B. W. et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 113, 321–327 (2014).
Marchetti, C. et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Wohlford, G. F. et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J. Cardiovasc. Pharmacol. 77, 49–60 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02272946 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03939520 (2022).
Martin, J. et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-β. Cardiovasc. Res. 65, 694–701 (2005).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03490708 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03923140 (2019).
Li, Y. et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol. Immunol. 99, 134–144 (2018).
Aimo, A. et al. Pirfenidone as a novel cardiac protective treatment. Heart Fail. Rev. 27, 525–532 (2022).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Lyu, H. et al. VX-765 prevents intestinal ischemia-reperfusion injury by inhibiting NLRP3 inflammasome. Tissue Cell 75, 101718 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05164120 (2022).
Toldo, S. et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 236, 108053 (2021).
Samuel, M. et al. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can. J. Cardiol. 37, 776–785 (2021).
Varghese, B. et al. Inflammation, atrial fibrillation, and the potential role for colchicine therapy. Heart Rhythm O2 2, 298–303 (2021).
Tabbalat, R. A. et al. Effect of low-dose colchicine on the incidence of atrial fibrillation in open heart surgery patients: END-AF low dose trial. J. Int. Med. Res. 48, 300060520939832 (2020).
Tabbalat, R. A. et al. Effect of colchicine on the incidence of atrial fibrillation in open heart surgery patients: END-AF trial. Am. Heart J. 178, 102–107 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03310125 (2022).
Aguilar, M., Heijman, J., Dobrev, D. & Nattel, S. One ring to rule them all: continuous monitoring of patients with secondary atrial fibrillation points to a unifying underlying mechanism. Can. J. Cardiol. 37, 686–689 (2021).
Tucker, N. R. et al. Transcriptional and cellular diversity of the human heart. Circulation 142, 466–482 (2020).
Moreira, L. M. et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 587, 460–465 (2020).
Litvinukova, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Zhang, Y. et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 118, 785–797 (2022).
Rong, J. et al. Loss of hepatic angiotensinogen attenuates sepsis-induced myocardial dysfunction. Circ. Res. 129, 547–564 (2021).
Shi, H. et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res. 129, 383–396 (2021).
Takahashi, M. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biol. Pharm. Bull. 42, 518–523 (2019).
Walkey, A. J., Evans, S. R., Winter, M. R. & Benjamin, E. J. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest 149, 74–83 (2016).
Spodick, D. H. Frequency of arrhythmias in acute pericarditis determined by Holter monitoring. Am. J. Cardiol. 53, 842–845 (1984).
Talreja, D. R. et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 108, 1852–1857 (2003).
Syed, F. F. et al. Atrial fibrillation as a consequence of tuberculous pericardial effusion. Int. J. Cardiol. 158, 152–154 (2012).
Larsen, B. T. et al. Atrial giant cell myocarditis: a distinctive clinicopathologic entity. Circulation 127, 39–47 (2013).
Anderson, B. R., Silver, E. S., Richmond, M. E. & Liberman, L. Usefulness of arrhythmias as predictors of death and resource utilization in children with myocarditis. Am. J. Cardiol. 114, 1400–1405 (2014).
Blagova, O. V. et al. Myocardial biopsy in “idiopathic” atrial fibrillation and other arrhythmias: nosological diagnosis, clinical and morphological parallels, and treatment. J. Atr. Fibrillation 9, 24–30 (2016).
Subahi, A. et al. Impact of atrial fibrillation on patients hospitalized for acute myocarditis: insights from a nationally-representative United States cohort. Clin. Cardiol. 42, 26–31 (2019).
Rasal, G. et al. Arrhythmia spectrum and outcome in children with myocarditis. Ann. Pediatr. Cardiol. 14, 366–371 (2021).
Dunbar, D. N. et al. Intracavitary electrode catheter cardioversion of atrial tachyarrhythmias in the dog. J. Am. Coll. Cardiol. 7, 1015–1027 (1986).
Kumagai, K., Yamanouchi, Y., Tashiro, N., Hiroki, T. & Arakawa, K. Low energy synchronous transcatheter cardioversion of atrial flutter/fibrillation in the dog. J. Am. Coll. Cardiol. 16, 497–501 (1990).
Ali, I. M., Butler, C. K., Armour, J. A. & Murphy, D. A. Modification of supraventricular tachyarrhythmias by stimulating atrial neurons. Ann. Thorac. Surg. 50, 251–256 (1990).
Yamanouchi, Y., Kumagai, K., Tashiro, N., Hiroki, T. & Arakawa, K. Transesophageal low-energy synchronous cardioversion of atrial flutter/fibrillation in the dog. Am. Heart J. 123, 417–420 (1992).
Ortiz, J. et al. Mechanism of spontaneous termination of stable atrial flutter in the canine sterile pericarditis model. Circulation 88, 1866–1877 (1993).
Ortiz, J. et al. Atrial defibrillation using temporary epicardial defibrillation stainless steel wire electrodes: studies in the canine sterile pericarditis model. J. Am. Coll. Cardiol. 26, 1356–1364 (1995).
Cmolik, B. L. et al. Successful atrial defibrillation with very-low-energy shocks by means of temporary epicardial wire electrodes. J. Thorac. Cardiovasc. Surg. 111, 392–397 (1996). discussion 397-398.
Kumagai, K., Khrestian, C. & Waldo, A. L. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model. Insights into the mechanism of its maintenance. Circulation 95, 511–521 (1997).
Sokoloski, M. C. et al. Safety of transvenous atrial defibrillation: studies in the canine sterile pericarditis model. Circulation 96, 1343–1350 (1997).
Kumagai, K., Uno, K., Khrestian, C. & Waldo, A. L. Single site radiofrequency catheter ablation of atrial fibrillation: studies guided by simultaneous multisite mapping in the canine sterile pericarditis model. J. Am. Coll. Cardiol. 36, 917–923 (2000).
Becker, R. et al. Suppression of atrial fibrillation by multisite and septal pacing in a novel experimental model. Cardiovasc. Res. 54, 476–481 (2002).
Kumagai, K., Nakashima, H., Gondo, N. & Saku, K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 14, 880–884 (2003).
Goldstein, R. N., Khrestian, C., Carlsson, L. & Waldo, A. L. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J. Cardiovasc. Electrophysiol. 15, 1444–1450 (2004).
Ryu, K. et al. Characterization of the critical cycle length of a left atrial driver which causes right atrial fibrillatory conduction. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 3960–3963 (2004).
Ryu, K., Sahadevan, J., Khrestian, C. M., Stambler, B. S. & Waldo, A. L. Use of fast Fourier transform analysis of atrial electrograms for rapid characterization of atrial activation – implications for delineating possible mechanisms of atrial tachyarrhythmias. J. Cardiovasc. Electrophysiol. 17, 198–206 (2006).
Tselentakis, E. V., Woodford, E., Chandy, J., Gaudette, G. R. & Saltman, A. E. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J. Surg. Res. 135, 68–75 (2006).
Bui, H. M., Khrestian, C. M., Ryu, K., Sahadevan, J. & Waldo, A. L. Fixed intercaval block in the setting of atrial fibrillation promotes the development of atrial flutter. Heart Rhythm. 5, 1745–1752 (2008).
Ryu, K., Sahadevan, J., Khrestian, C. M., Stambler, B. S. & Waldo, A. L. Frequency analysis of atrial electrograms identifies conduction pathways from the left to the right atrium during atrial fibrillation – studies in two canine models. J. Cardiovasc. Electrophysiol. 20, 667–674 (2009).
Rossman, E. I. et al. The gap junction modifier, GAP-134 [(2S,4R)-1-(2-aminoacetyl)-4-benzamido-pyrrolidine-2-carboxylic acid], improves conduction and reduces atrial fibrillation/flutter in the canine sterile pericarditis model. J. Pharmacol. Exp. Ther. 329, 1127–1133 (2009).
Matsumoto, N. et al. Vanoxerine, a new drug for terminating atrial fibrillation and flutter. J. Cardiovasc. Electrophysiol. 21, 311–319 (2010).
Cakulev, I. et al. Oral vanoxerine prevents reinduction of atrial tachyarrhythmias: preliminary results. J. Cardiovasc. Electrophysiol. 22, 1266–1273 (2011).
Bhimani, A. A. et al. Ranolazine terminates atrial flutter and fibrillation in a canine model. Heart Rhythm. 11, 1592–1599 (2014).
Sadrpour, S. A. et al. Termination of atrial flutter and fibrillation by K201’s metabolite M-II: studies in the canine sterile pericarditis model. J. Cardiovasc. Pharmacol. 65, 494–499 (2015).
Goldstein, R. N., Ryu, K., Khrestian, C., van Wagoner, D. R. & Waldo, A. L. Prednisone prevents inducible atrial flutter in the canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 19, 74–81 (2008).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04575753 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04997057 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03609541 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04697719 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02322073 (2021).
Acknowledgements
The authors thank T. Poppenborg (Institute of Pharmacology, University Duisburg-Essen, Germany) for help with preparing the figures for initial submission and A. Saljic (Institute of Pharmacology, University Duisburg-Essen, Germany) for critical review of the tables. The authors are supported by funding from the National Institutes of Health (R01HL136389, R01HL131517, R01HL089598 and R01HL163277 to D.D.; R01HL136389, R01HL147108 and R01HL163277 to N.L.), the German Research Foundation (DFG, Do 769/4-1 to D.D.), the European Union (large-scale integrative project MAESTRIA, no. 965286 to D.D.), the Netherlands Organization for Scientific Research (NWO/ZonMW Vidi 09150171910029 to J.H.), the Montreal Heart Institute Foundation (4800 to R.H.), the Canada Foundation for Innovation (42228 to S.N. and R.H.), the Canadian Institutes of Health Research (148401 to S.N.), and the AHA (Established Investigator Award 936111 to N.L).
Author information
Authors and Affiliations
Contributions
All the authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Antonio Abbate, Shih-Ann Chen and David van Wagoner for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dobrev, D., Heijman, J., Hiram, R. et al. Inflammatory signalling in atrial cardiomyocytes: a novel unifying principle in atrial fibrillation pathophysiology. Nat Rev Cardiol 20, 145–167 (2023). https://doi.org/10.1038/s41569-022-00759-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-022-00759-w
This article is cited by
-
Evaluating the risk of atrial fibrillation in patients with chronic recurrent pericarditis prescribed colchicine: Observations using TriNetX global federated research network
European Journal of Clinical Pharmacology (2026)
-
Association between fibrinogen-to-albumin ratio and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study using the MIMIC-IV database
European Journal of Medical Research (2025)
-
Prognostic value of hemoglobin, albumin, lymphocyte, platelet (HALP) scores in patients with non-valvular atrial fibrillation: insights from the AFTER-2 study
BMC Cardiovascular Disorders (2025)
-
ETV1 orchestrates a self-reinforcing ERK1/2-RSK3 signaling loop to drive atrial fibrillation pathogenesis: implications for targeted therapy
Scientific Reports (2025)
-
Atrial fibrillation risk model based on LASSO and SVM algorithms and immune infiltration of key mitochondrial energy metabolism genes
Scientific Reports (2025)