Abstract
The gut microbiota has emerged as an environmental risk factor that affects thrombotic phenotypes in several cardiovascular diseases. Evidence includes the identification of marker species by sequencing studies of the gut microbiomes of patients with thrombotic disease, the influence of antithrombotic therapies on gut microbial diversity, and preclinical studies in mouse models of thrombosis that have demonstrated the functional effects of the gut microbiota on vascular inflammatory phenotypes and thrombus formation. In addition to impaired gut barrier function promoting low-grade inflammation, gut microbiota-derived metabolites have been shown to act on vascular cell types and promote thrombus formation. Therefore, these meta-organismal pathways that link the metabolic capacities of gut microorganisms with host immune functions have emerged as potential diagnostic markers and novel drug targets. In this Review, we discuss the link between the gut microbiota, its metabolites and thromboembolic diseases.
Key points
-
Gut microbial composition is associated with thromboembolic disease states.
-
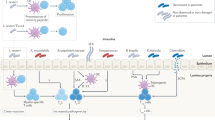
Intestinal barrier function is crucial to prevent the translocation of microbial components from the gut lumen into the blood circulation, including microbial patterns that can activate innate immune pathways and affect thrombosis potential via the gut–liver axis.
-
Gut microbial metabolites can influence vascular cells and myeloid cells, affecting platelet responsiveness and cellular interactions with the vessel wall.
-
Well-studied examples of gut microbial metabolites include choline-derived and carnitine-derived trimethylamine N-oxide, essential amino acid-derived metabolites (indoxyl sulfate, p-cresol sulfate and phenylacetylglutamine) and dietary fibre-derived short-chain fatty acids.
-
The gut microbiota and its metabolites are promising diagnostic markers and potential novel targets for the prevention and treatment of thromboembolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mackman, N. Triggers, targets and treatments for thrombosis. Nature 451, 914–918 (2008).
Münzel, T. et al. Heart healthy cities: genetics loads the gun but the environment pulls the trigger. Eur. Heart J. 42, 2422–2438 (2021).
Enav, H., Bäckhed, F. & Ley, R. E. The developing infant gut microbiome: a strain-level view. Cell Host Microbe 30, 627–638 (2022).
Esser, D. et al. Functions of the microbiota for the physiology of animal metaorganisms. J. Innate Immun. 11, 393–404 (2019).
Wu, M. et al. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell 187, 897–913.e18 (2024).
Motta, J. P. et al. Active thrombin produced by the intestinal epithelium controls mucosal biofilms. Nat. Commun. 10, 3224 (2019).
Jäckel, S. et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood 130, 542–553 (2017).
Sommer, F. & Bäckhed, F. The gut microbiota – masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Gola, A. et al. Commensal-driven immune zonation of the liver promotes host defence. Nature 589, 131–136 (2021).
Formes, H. et al. The gut microbiota instructs the hepatic endothelial cell transcriptome. iScience 24, 103092 (2021).
Zhu, W. et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124 (2016).
Witkowski, M. et al. Vascular endothelial tissue factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc. Res. 118, 2367–2384 (2022).
Carnevale, R. et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: implication for myocardial infarction. Eur. Heart J. 41, 3156–3165 (2020).
Skye, S. M. et al. Microbial transplantation with human gut commensals containing cutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ. Res. 123, 1164–1176 (2018).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Nemet, I. et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 180, 862–877.e22 (2020).
Nemet, I. et al. Microbe-derived uremic solutes enhance thrombosis potential in the host. mBio 14, e0133123 (2023).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Schaupp, L. et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell 181, 1080–1096.e19 (2020).
Reininger, A. J. et al. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J. Am. Coll. Cardiol. 55, 1147–1158 (2010).
Oppi, S., Luscher, T. F. & Stein, S. Mouse models for atherosclerosis research – which is my line? Front. Cardiovasc. Med. 6, 46 (2019).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Kiouptsi, K. et al. The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice. mBio 10, e02298-19 (2019).
Frost, F. et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: a pilot study. PLoS ONE 14, e0219489 (2019).
Karlsson et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3, 1245 (2012).
Jie, Z. et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8, 845 (2017).
Jie, Z. et al. A consortium of three-bacteria isolated from human feces inhibits formation of atherosclerotic deposits and lowers lipid levels in a mouse model. iScience 26, 106960 (2023).
Ferrell, M. et al. Fecal microbiome composition does not predict diet-induced TMAO production in healthy adults. J. Am. Heart Assoc. 10, e021934 (2021).
Ott, S. J. et al. Fungal rDNA signatures in coronary atherosclerotic plaques. Env. Microbiol. 9, 3035–3045 (2007).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl Acad. Sci. USA 108, 4592–4598 (2011).
Lehtiniemi, J., Karhunen, P. J., Goebeler, S., Nikkari, S. & Nikkari, S. T. Identification of different bacterial DNAs in human coronary arteries. Eur. J. Clin. Invest. 35, 13–16 (2005).
Ott, S. J. et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 113, 929–937 (2006).
Lindskog Jonsson, A. et al. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 263, 177–183 (2017).
Yang, M. et al. Large-scale correlation analysis of deep venous thrombosis and gut microbiota. Front. Cardiovasc. Med. 9, 1025918 (2022).
Shannon, O. et al. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol. Microbiol. 65, 1147–1157 (2007).
Kiouptsi, K., Pontarollo, G. & Reinhardt, C. Gut microbiota and the microvasculature. Cold Spring Harb. Perspect. Med. 13, a041179 (2023).
Stappenbeck, T. S., Hooper, L. V. & Gordon, J. I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl Acad. Sci. USA 99, 15451–15455 (2002).
Reinhardt, C. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2012).
Suh, S. H. et al. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep. 20, e46927 (2019).
Tang, A. T. et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 545, 305–310 (2017).
Komatsu, S., Berg, R. D., Russell, J. M., Nimura, Y. & Granger, D. N. Enteric microflora contribute to constitutive ICAM-1 expression on vascular endothelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G186–G191 (2000).
Balmer, M. L. et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 193, 5273–5283 (2014).
Zhang, D. et al. Neutrophil ageing is regulated by the microbiome. Nature 525, 528–532 (2015).
Clarke, T. B. et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 (2010).
Haghikia, A. et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler. Thromb. Vasc. Biol. 38, 2225–2235 (2018).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Deppermann, C. et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J. Clin. Invest. 123, 3331–3342 (2013).
Roewe, J. et al. Bacterial polyphosphates interfere with the innate host defense to infection. Nat. Commun. 11, 4035 (2020).
Su, L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563 (2009).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Zhou, X. et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 6, 66 (2018).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Vieira-Silva, S. et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315 (2020).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e4 (2017).
Inczefi, O. et al. Targeted intestinal tight junction hyperpermeability alters the microbiome, behavior, and visceromotor responses. Cell Mol. Gastroenterol. Hepatol. 10, 206–208.e3 (2020).
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Franchimont, D. et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut 53, 987–992 (2004).
Hayes, C. L. et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci. Rep. 8, 14184 (2018).
Pontarollo, G. et al. Commensal bacteria weaken the intestinal barrier by suppressing epithelial neuropilin-1 and Hedgehog signaling. Nat. Metab. 5, 1174–1187 (2023).
Wiedermann, C. J. et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J. Am. Coll. Cardiol. 34, 1975–1981 (1999).
Carnevale, R. et al. Localization of lipopolysaccharide from Escherichia coli into human atherosclerotic plaque. Sci. Rep. 8, 3598 (2018).
Liu, C. et al. Circulating LPS from gut microbiota leverages stenosis-induced deep vein thrombosis in mice. Thromb. J. 21, 71 (2023).
Aburima, A. et al. Thrombospondin-1 promotes hemostasis through modulation of cAMP signaling in blood platelets. Blood 137, 678–689 (2021).
Semeraro, N. et al. Direct induction of tissue factor synthesis by endotoxin in human macrophages from diverse anatomical sites. Immunology 50, 529–535 (1983).
Carnevale, R. et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J. Hepatol. 67, 950–956 (2017).
Biswas, S., Zimman, A., Gao, D., Byzova, T. V. & Podrez, E. A. TLR2 plays a key role in platelet hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ. Res. 121, 951–962 (2017).
Blair, P. et al. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ. Res. 104, 346–354 (2009).
Gerold, G. et al. A toll-like receptor 2-integrin β3 complex senses bacterial lipopeptides via vitronectin. Nat. Immunol. 9, 761–768 (2008).
Parra-Izquierdo, I. et al. The toll-like receptor 2 ligand Pam2CSK4 activates platelet nuclear factor-κB and Bruton’s tyrosine kinase signaling to promote platelet-endothelial cell interactions. Front. Immunol. 12, 729951 (2021).
Li, G. et al. Gut microbiota aggravates neutrophil extracellular traps-induced pancreatic injury in hypertriglyceridemic pancreatitis. Nat. Commun. 14, 6179 (2023).
Ma, Y. et al. Mechanism of taurine reducing inflammation and organ injury in sepsis mice. Cell Immunol. 375, 104503 (2022).
Ascher, S. et al. Gut microbiota restricts NETosis in acute mesenteric ischemia-reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 40, 2279–2292 (2020).
Parker, L. C. et al. Endotoxin tolerance induces selective alterations in neutrophil function. J. Leukoc. Biol. 78, 1301–1305 (2005).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Witkowski, M., Weeks, T. L. & Hazen, S. L. Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570 (2020).
Zhu, Y. et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 31, 18–32.e9 (2023).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Koeth, R. A. et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 129, 373–387 (2019).
Martinez-del Campo, A. et al. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 6, e00042-15 (2015).
Koeth, R. A. et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to TMAO. Cell Metab. 20, 799–812 (2014).
Buffa, J. A. et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota l-carnitine catabolism. Nat. Microbiol. 7, 73–86 (2022).
Romano, K. A., Vivas, E. I., Amador-Noguez, D. & Rey, F. E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6, e02481 (2015).
Rath, S., Heidrich, B., Pieper, D. H. & Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 5, 54 (2017).
Seim, H., Löster, H., Claus, R., Kleber, H.-P. & Strack, E. Splitting of the C–N bond in carnitine by an enzyme (trimethylamine forming) from membranes of Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 15, 165–167 (1982).
Unemoto, T., Hayashi, M., Miyaki, K. & Hayashi, M. Formation of trimethylamine from dl-carnitine by Serratia marcescens. Biochim. Biophys. Acta 121, 220–222 (1966).
Bennett, B. J. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60 (2013).
Aldana-Hernandez, P. et al. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr−/− and Apoe−/− male mice. J. Nutr. 150, 249–255 (2020).
Lindskog Jonsson, A. et al. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 38, 2318–2326 (2018).
Koay, Y. C. et al. Plasma levels of trimethylamine-N-oxide can be increased with ‘healthy’ and ‘unhealthy’ diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc. Res. 117, 435–449 (2021).
Huang, K. et al. Gut microbial co-metabolite 2-methylbutyrylcarnitine exacerbates thrombosis via binding to and activating integrin α2β1. Cell Metab. 36, 598–616.e9 (2024).
Emonds, J. J. et al. Influence of trimethylamine N-oxide on platelet activation. Nutrients 14, 3261 (2022).
Zhu, W. et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 29, 1199–1208.e5 (2021).
Sun, X. et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 481, 63–70 (2016).
Ma, G. et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 37, BSR20160244 (2017).
Cybulsky, M. I. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107, 1255–1262 (2001).
Querio, G., Antoniotti, S., Geddo, F., Levi, R. & Gallo, M. P. Trimethylamine N-oxide (TMAO) impairs purinergic induced intracellular calcium increase and nitric oxide release in endothelial cells. Int. J. Mol. Sci. 23, 3982 (2022).
Ren, D., Liu, Y., Zhao, Y. & Yang, X. Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem. Toxicol. 94, 203–212 (2016).
Jomard, A. et al. Effects of acute administration of trimethylamine N-oxide on endothelial function: a translational study. Sci. Rep. 12, 8664 (2022).
Wang, M. et al. Trimethylamine N-oxide is associated with long-term mortality risk: the multi-ethnic study of atherosclerosis. Eur. Heart J. 44, 1608–1618 (2023).
Yin, J. et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 4, e002699 (2015).
Ringel, C. et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis 335, 62–67 (2021).
Wang, M. et al. Dietary meat, trimethylamine N-oxide-related metabolites, and incident cardiovascular disease among older adults: the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 42, e273–e288 (2022).
Lemaitre, R. N. et al. Plasma trimethylamine-N-oxide and incident ischemic stroke: the Cardiovascular Health Study and the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 12, e8711 (2023).
Tan, Y. et al. Association between plasma trimethylamine N-oxide and neoatherosclerosis in patients with very late stent thrombosis. Can. J. Cardiol. 36, 1252–1260 (2020).
Liu, X. et al. Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int. J. Cardiol. 265, 18–23 (2018).
Fu, Q. et al. Coronary plaque characterization assessed by optical coherence tomography and plasma trimethylamine-N-oxide levels in patients with coronary artery disease. Am. J. Cardiol. 118, 1311–1315 (2016).
Bordoni, L. et al. Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: a cross-sectional study. Sci. Rep. 10, 18675 (2020).
Zhu, W., Wang, Z., Tang, W. H. W. & Hazen, S. L. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation 135, 1671–1673 (2017).
Yu, F. et al. Phenylacetylglutamine, a novel biomarker in acute ischemic stroke. Front. Cardiovasc. Med. 8, 798765 (2021).
Fang, C. et al. Dysbiosis of gut microbiota and metabolite phenylacetylglutamine in coronary artery disease patients with stent stenosis. Front. Cardiovasc. Med. 9, 832092 (2022).
Romano, K. A. et al. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ. Heart Fail. 16, e009972 (2023).
Kim, D. K. et al. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J. Biol. Chem. 276, 17221–17228 (2001).
Chen, C., Yin, Y., Tu, Q. & Yang, H. Glucose and amino acid in enterocyte: absorption, metabolism and maturation. Front. Biosci. 23, 1721–1739 (2018).
Asano, Y. et al. Phenylalanine dehydrogenase of Bacillus badius. Purification, characterization and gene cloning. Eur. J. Biochem. 168, 153–159 (1987).
Mavrides, C. & Orr, W. Multispecific aspartate and aromatic amino acid aminotransferases in Escherichia coli. J. Biol. Chem. 250, 4128–4133 (1975).
Mayrand, D. Identification of clinical isolates of selected species of Bacteroides: production of phenylacetic acid. Can. J. Microbiol. 25, 927–928 (1979).
Russell, W. R. et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res. 57, 523–535 (2013).
Feng, R. et al. Gut microbiome-generated phenylacetylglutamine from dietary protein is associated with Crohn’s disease and exacerbates colitis in mouse model possibly via platelet activation. J. Crohns Colitis 17, 1833–1846 (2023).
Webster, L. T., Siddiqui, U. A., Lucas, S. V., Strong, J. M. & Mieyal, J. J. Identification of separate acyl-CoA:glycine and acyl-CoA:L-glutamine N-acyltransferase activities in mitochondrial fractions from liver of rhesus monkey and man. J. Biol. Chem. 251, 3352–3358 (1976).
Paeslack, N. et al. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 54, 1339–1356 (2022).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Lee, J. H., Wood, T. K. & Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 23, 707–718 (2015).
Barreto, F. C. et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4, 1551–1558 (2009).
Nemet, I. et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur. Heart J. 44, 3085–3096 (2023).
Adijiang, A., Goto, S., Uramoto, S., Nishijima, F. & Niwa, T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transpl. 23, 1892–1901 (2008).
Wu, C. C. et al. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J. Am. Soc. Nephrol. 27, 1254–1264 (2016).
Yang, K. et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood 129, 2667–2679 (2017).
Karbowska, M. et al. The uremic toxin indoxyl sulfate accelerates thrombotic response after vascular injury in animal models. Toxins 9, 229 (2017).
Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 72, 1–11 (2010).
Dou, L. et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 5, 1302–1308 (2007).
Xu, K. et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut https://doi.org/10.1136/gutjnl-2020-323263 (2021).
Ito, S. et al. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J. Biol. Chem. 285, 38869–38875 (2010).
Gondouin, B. et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 84, 733–744 (2013).
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Martinez, A. W., Recht, N. S., Hostetter, T. H. & Meyer, T. W. Removal of p-cresol sulfate by hemodialysis. J. Am. Soc. Nephrol. 16, 3430–3436 (2005).
Saito, Y., Sato, T., Nomoto, K. & Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 94, fly125 (2018).
Gryp, T., Vanholder, R., Vaneechoutte, M. & Glorieux, G. p-Cresyl sulfate. Toxins (Basel) 9, 52 (2017).
Gross, P. et al. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J. Cell Physiol. 230, 2927–2935 (2015).
Jing, Y. J. et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE−/− mice. Kidney Int. 89, 439–449 (2016).
Cummings, J. H. Fermentation in the human large intestine: evidence and implications for health. Lancet 1, 1206–1209 (1983).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Rey, F. E. et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 285, 22082–22090 (2010).
Miller, T. L. & Wolin, M. J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Env. Microbiol. 62, 1589–1592 (1996).
Reichardt, N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8, 1323–1335 (2014).
Scott, K. P., Martin, J. C., Campbell, G., Mayer, C. D. & Flint, H. J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 188, 4340–4349 (2006).
Vital, M., Howe, A. C. & Tiedje, J. M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 5, e00889 (2014).
Aguilar, E. C. et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 24, 606–613 (2014).
Aguilar, E. C. et al. Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J. Nutr. Biochem. 34, 99–105 (2016).
Kasahara, K. et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 3, 1461–1471 (2018).
Liu, H. et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 7, 68 (2019).
Dremova, O. et al. Sterility testing of germ-free mouse colonies. Front. Immunol. 14, 1275109 (2023).
Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W. & Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 133, 2434–2446 (2016).
Brandsma, E. et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 124, 94–100 (2019).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Dürholz, K. et al. Microbiota-derived propionate modulates megakaryopoiesis and platelet function. Front. Immunol. 13, 908174 (2022).
Fukumoto, S. et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1269–R1276 (2003).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Kamp, M. E. et al. G protein-coupled receptor 43 modulates neutrophil recruitment during acute inflammation. PLoS ONE 11, e0163750 (2016).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Haghikia, A. et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 43, 518–533 (2022).
Garber, K. Drugging the gut microbiome. Nat. Biotechnol. 33, 228–231 (2015).
de Groot, P. et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 70, 92–105 (2021).
Roubaud-Baudron, C. et al. Long-term effects of early-life antibiotic exposure on resistance to subsequent bacterial infection. mBio 10, e02820-19 (2019).
Feuerstadt, P. et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N. Engl. J. Med. 386, 220–229 (2022).
Carvalho, T. First oral fecal microbiota transplant therapy approved. Nat. Med. 29, 1581–1582 (2023).
Barmparas, G. et al. Clostridium difficile increases the risk for venous thromboembolism. Am. J. Surg. 208, 703–709 (2014).
Kumar, A., Ghazanfar, H. & Davidson, J. M. A rare case of arterial and venous thromboembolism in a patient with severe Clostridium difficile infection. Cureus 13, e16103 (2021).
Kim, E. S. et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp. Mol. Med. 54, 103–114 (2022).
Mohammed, Y. et al. The intestinal microbiome potentially affects thrombin generation in human subjects. J. Thromb. Haemost. 18, 642–650 (2020).
Ott, S. J. et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 152, 799–811.e7 (2017).
Zuo, T. et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 67, 634–643 (2018).
DeFilipp, Z. et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 381, 2043–2050 (2019).
Smits, L. P. et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J. Am. Heart Assoc. 7, e008342 (2018).
Wang, Z. et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163, 1585–1595 (2015).
Roberts, A. B. et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 24, 1407–1417 (2018).
Quareshy, M. et al. Structural basis of carnitine monooxygenase CntA substrate specificity, inhibition, and intersubunit electron transfer. J. Biol. Chem. 296, 100038 (2021).
Collins, H. L. et al. l-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis 244, 29–37 (2016).
Shi, W., Huang, Y., Yang, Z., Zhu, L. & Yu, B. Reduction of TMAO level enhances the stability of carotid atherosclerotic plaque through promoting macrophage M2 polarization and efferocytosis. Biosci. Rep. 41, BSR20204250 (2021).
Mitchell, S. C. & Smith, R. L. Trimethylaminuria: the fish malodor syndrome. Drug. Metab. Dispos. 29, 517–521 (2001).
Benson, T. W. et al. Gut microbiota-derived trimethylamine N-oxide contributes to abdominal aortic aneurysm through inflammatory and apoptotic mechanisms. Circulation 147, 1079–1096 (2023).
Vilskersts, R. et al. Myocardial infarct size-limiting and anti-arrhythmic effects of mildronate orotate in the rat heart. Cardiovasc. Drugs Ther. 23, 281–288 (2009).
Chen, S. et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 30, 1141–1151.e5 (2019).
Nakabayashi, I. et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol. Dial. Transpl. 26, 1094–1098 (2011).
Donckier, J., Anderson, J. V., Yeo, T. & Bloom, S. R. Diurnal rhythm in the plasma concentration of atrial natriuretic peptide. N. Engl. J. Med. 315, 710–711 (1986).
Graboski, A. L. et al. Mechanism-based inhibition of gut microbial tryptophanases reduces serum indoxyl sulfate. Cell Chem. Biol. 30, 1402–1413.e7 (2023).
Lee, C. T. et al. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 37, 76–83 (2014).
Yamamoto, S. et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol. Dial. Transpl. 26, 2491–2497 (2011).
Anderson, R. C. et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 10, 316 (2010).
Zhao, X. et al. Probiotics mixture reinforces barrier function to ameliorate necrotizing enterocolitis by regulating PXR-JNK pathway. Cell Biosci. 11, 20 (2021).
Souza, D. G., Senchenkova, E. Y., Russell, J. & Granger, D. N. MyD88 mediates the protective effects of probiotics against the arteriolar thrombosis and leukocyte recruitment associated with experimental colitis. Inflamm. Bowel Dis. 21, 888–900 (2015).
Ali, F. Y. et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler. Thromb. Vasc. Biol. 29, 706–711 (2009).
Undas, A., Brummel, K. E., Musial, J., Mann, K. G. & Szczeklik, A. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation 103, 2248–2253 (2001).
Colli, S. et al. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 17, 265–272 (1997).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4, 1826–1831 (2019).
Wilmanski, T. et al. Heterogeneity in statin responses explained by variation in the human gut microbiome. Med 3, 388–405.e6 (2022).
Wang, L. et al. The gut microbes, Enterococcus and Escherichia-Shigella, affect the responses of heart valve replacement patients to the anticoagulant warfarin. Pharmacol. Res. 159, 104979 (2020).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260 (2018).
Zhang, X. et al. Gut microbiota induces high platelet response in patients with ST segment elevation myocardial infarction after ticagrelor treatment. Elife 11, e70240 (2022).
Guasch-Ferre, M. et al. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention With Mediterranean Diet) study. J. Am. Heart Assoc. 6, e006524 (2017).
Mueller, D. M. et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 243, 638–644 (2015).
Senthong, V. et al. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J. Am. Heart Assoc. 5, e004237 (2016).
Matsuzawa, Y. et al. Microbiota-derived trimethylamine N-oxide predicts cardiovascular risk after STEMI. Sci. Rep. 9, 11647 (2019).
Han, Y. et al. Dysbiosis of gut microbiota in patients with acute myocardial infarction. Front. Microbiol. 12, 680101 (2021).
Raju, S. C. et al. Microbial-derived imidazole propionate links the heart failure-associated microbiome alterations to disease severity. Genome Med. 16, 27 (2024).
Liang, Z. et al. Trimethylamine N-oxide as a risk marker for ischemic stroke in patients with atrial fibrillation. J. Biochem. Mol. Toxicol. 33, e22246 (2019).
Luciani, M. et al. Trimethylamine-N-oxide is associated with cardiovascular mortality and vascular brain lesions in patients with atrial fibrillation. Heart 109, 396–404 (2023).
Meyer, K. A. et al. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 5, e003970 (2016).
Kijpaisalratana, N. et al. Trimethylamine N-oxide and white matter hyperintensity volume among patients with acute ischemic stroke. JAMA Netw. Open. 6, e2330446 (2023).
Gagne, M. A. et al. Dysbiotic microbiota contributes to the extent of acute myocardial infarction in rats. Sci. Rep. 12, 16517 (2022).
Chen, C., Zhang, H., Xie, R., Wang, Y. & Ma, Y. Gut microbiota aggravate cardiac ischemia-reperfusion injury via regulating the formation of neutrophils extracellular traps. Life Sci. 303, 120670 (2022).
Moludi, J. et al. Probiotics supplementation on cardiac remodeling following myocardial infarction: a single-center double-blind clinical study. J. Cardiovasc. Transl. Res. 14, 299–307 (2021).
Lopez-Aladid, R. et al. Determining the most accurate 16S rRNA hypervariable region for taxonomic identification from respiratory samples. Sci. Rep. 13, 3974 (2023).
Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J. & Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 35, 833–844 (2017).
Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 13, 423–434 (2020).
Acknowledgements
M.P.K., N.P., and O.D. are PhD students at the Mainz Research School of Translational Biomedicine (TransMed) and members of Young DZHK. K.K. is supported by the German Center for Cardiovascular Research (DZHK) “Promotion of women scientists” Excellence Programme and is a member of Young DZHK. C.R. acknowledges funding from the Forschungsinitiative Rheinland-Pfalz and ReALity (project MORE), the Wilhelm Sander-Stiftung (Nr. 2022.131.1), the BMBF Cluster4Future CurATime (project MicrobAIome, 03ZU1202CA) and the Deutsche Zentren der Gesundheitsforschung (DZG) Innovation Fund “Microbiome” (81X2210129); is a scientist at DZHK; is a member of the Center for Translational Vascular Biology (CTVB), the Research Center for Immunotherapy (FZI), the Potentialbereich EXPOHEALTH at the Johannes Gutenberg-University Mainz, and the DFG Research Unit 5644 INFINITE (RE 3450/15-1); and was awarded a Fellowship from the Gutenberg Research College at the Johannes Gutenberg-University Mainz.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Kazuyuki Kasahara and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Altered Schaedler flora
-
Minimal gut microbial consortium composed of eight defined common bacterial species found in mice.
- Endothelial cell adhesion molecules
-
Cell surface proteins expressed on endothelial cells that mediate the adhesion and infiltration of leukocytes and other blood cells; these adhesion molecules can be divided into integrins, selectins and immunoglobulin superfamily members.
- Germ-free mouse models
-
Mice lacking all microorganisms and housed in a sterile environment; this model is used to study gut microbiota–host interactions.
- Gnotobiotic
-
An engineered state of an organism in which all forms of life in or on it, including the gut microbiota, have been identified; model organisms can be colonized with a specific community of known microorganisms or can contain no microorganisms (germ-free).
- Gut–liver axis
-
Bidirectional relationship between the intestine, including the gut microbiota, and the liver.
- Immunothrombosis
-
Physiological host response to invading pathogens, involving the recruitment of innate immune cells and activation of the coagulation cascade; monocytes and neutrophils trigger thrombus formation, which impedes the spread and invasion of pathogens.
- Low-grade endotoxaemia
-
Low levels of circulating endotoxins that influence inflammatory and metabolic conditions.
- Microbiome
-
Collection of all microorganisms and their genomic content in a defined environment.
- Microbiota
-
Collection of all microorganisms, including eukaryotes, archaea, bacteria and viruses, inhabiting a defined environment, such as a host or a specific niche.
- Mutualistic
-
Describing a beneficial relationship between different organisms.
- Pathogen-associated molecular patterns
-
Structural motifs conserved within a group of related microorganisms that are recognized by receptors of the innate immune system.
- Prebiotic
-
Nutritional supplement that nurtures specific groups of beneficial microorganisms.
- Probiotic
-
Selected and living microorganisms that are intended to confer health benefits to consumers if consumed in sufficient amounts.
- ST-segment elevation myocardial infarction
-
Myocardial infarction caused by the occlusion of a coronary artery and associated with ST-segment elevation on an electrocardiogram.
- Synbiotics
-
Combination of selected living microorganisms (probiotics) and nutritional substrates (prebiotics) that are intended to confer a health benefit to the host.
- T helper 17 cells
-
Lineage of T helper cells that can express various cytokines, such as IL-17, and which are involved in host defence and autoimmune diseases.
- Thromboinflammation
-
Pathological process in which inflammatory and thrombotic pathways are dysregulated, contributing to tissue damage.
- Thrombosis
-
The pathological formation of a blood clot in a vessel, which can obstruct the blood flow.
- Tissue factor
-
A primary initiator of the coagulation cascade and a member of the type 2 cytokine receptor family (extrinsic coagulation pathway).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khuu, M.P., Paeslack, N., Dremova, O. et al. The gut microbiota in thrombosis. Nat Rev Cardiol 22, 121–137 (2025). https://doi.org/10.1038/s41569-024-01070-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-024-01070-6
This article is cited by
-
Moderating role of blood inflammatory markers in the vaginal microbiota-fibrinogen relationship in URSA-affected women
Scientific Reports (2026)
-
The gut metabolite imidazole propionate is a potential biomarker of and therapeutic target for atherosclerosis
Nature Reviews Cardiology (2025)
-
Metabolic reprogramming in ischemic stroke: when glycolytic overdrive meets lipid storm
Cell Death & Disease (2025)
-
Gut microbiome and inflammation in cardiovascular drug response: trends in therapeutic success and commercial focus
Inflammopharmacology (2025)