Abstract
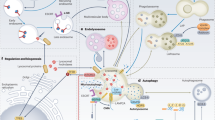
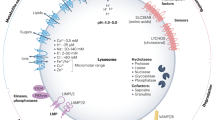
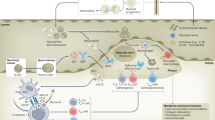
Lysosomes have a central role in the disposal of extracellular and intracellular cargo and also function as metabolic sensors and signalling platforms in the immunometabolic reprogramming of macrophages and other immune cells in atherosclerosis. Lysosomes can rapidly sense the presence of nutrients within immune cells, thereby switching from catabolism of extracellular material to the recycling of intracellular cargo. Such a fine-tuned degradative response supports the generation of metabolic building blocks through effectors such as mTORC1 or TFEB. By coupling nutrients to downstream signalling and metabolism, lysosomes serve as a crucial hub for cellular function in innate and adaptive immune cells. Lysosomal dysfunction is now recognized to be a hallmark of atherogenesis. Perturbations in nutrient-sensing and signalling have profound effects on the capacity of immune cells to handle cholesterol, perform phagocytosis and efferocytosis, and limit the activation of the inflammasome and other inflammatory pathways. Strategies to improve lysosomal function hold promise as novel modulators of the immunoinflammatory response associated with atherosclerosis. In this Review, we describe the crosstalk between lysosomal biology and immune cell function and polarization, with a particular focus on cellular immunometabolic reprogramming in the context of atherosclerosis.
Key points
-
Lysosomes are centralized hubs for metabolic sensing and functional reprogramming of cells.
-
Lysosomal metabolic sensing governs immune cell homeostasis and function.
-
Lysosome dysfunction contributes to the immunoinflammatory response and metabolic impairment in vascular atherosclerotic lesions.
-
Lysosomes are a compelling target for the modulation of immune responses in atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mony, V. K., Benjamin, S. & O’Rourke, E. J. A lysosome-centered view of nutrient homeostasis. Autophagy 12, 619–631 (2016).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020). A review discussing the intimate association between lysosomes and mTORC that is required to regulate cellular metabolic fate in states of nutrition or starvation in health and disease.
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 (2018).
de Duve, C. The participation of lysosomes in the transformation of smooth muscle cells to foamy cells in the aorta of cholesterol-fed rabbits. Acta Cardiol. 20, 9–25 (1974). Recognition of atherosclerotic plaque as a form of LSD as a consequence of neutral lipid deposition in lysosomes of foam cells.
Bar-Peled, L. & Sabatini, D. M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406 (2014).
Meng, Y., Heybrock, S., Neculai, D. & Saftig, P. Cholesterol handling in lysosomes and beyond. Trends Cell Biol. 30, 452–466 (2020).
Zhang, X. et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat. Metab. 2, 110–125 (2020).
Zernecke, A. et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 127, 402–426 (2020).
Chinetti-Gbaguidi, G., Colin, S. & Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 12, 10–17 (2015).
Wculek, S. K., Dunphy, G., Heras-Murillo, I., Mastrangelo, A. & Sancho, D. Metabolism of tissue macrophages in homeostasis and pathology. Cell. Mol. Immunol. 19, 384–408 (2022).
O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19, 273–281 (2019).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Nomura, M. et al. Fatty acid oxidation in macrophage polarization. Nat. Immunol. 17, 216–217 (2016).
Huang, S. C. C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15, 846–855 (2014).
Xu, R. et al. Lipid-associated macrophages between aggravation and alleviation of metabolic diseases. Trends Endocrinol. Metab. https://doi.org/10.1016/j.tem.2024.04.009 (2024).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178, 686–698.e14 (2019).
Dib, L. et al. Lipid-associated macrophages transition to an inflammatory state in human atherosclerosis, increasing the risk of cerebrovascular complications. Nat. Cardiovasc. Res. 2, 656–672 (2023).
Viaud, M. et al. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circ. Res. 122, 1369–1384 (2018).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Yan, C. et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am. J. Pathol. 169, 916–926 (2006).
Li, F. et al. Hepatic lysosomal acid lipase drives the autophagy-lysosomal response and alleviates cholesterol metabolic disorder in ApoE deficient mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 159027 (2021).
Schott, M. B. et al. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 218, 3320–3335 (2019).
Ouimet, M. et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 13, 655–667 (2011).
Tavakoli, S., Zamora, D., Ullevig, S. & Asmis, R. Bioenergetic profiles diverge during macrophage polarization: implications for the interpretation of 18F-FDG PET imaging of atherosclerosis. J. Nucl. Med. 54, 1661–1667 (2013).
Susser, L. I. et al. Mitochondrial fragmentation promotes inflammation resolution responses in macrophages via histone lactylation. Mol. Cell. Biol. 43, 531–546 (2023).
Afroz, S. F. et al. Mitochondrial dynamics in macrophages: divide to conquer or unite to survive? Biochem. Soc. Trans. 51, 41–56 (2023).
Vergadi, E., Ieronymaki, E., Lyroni, K., Vaporidi, K. & Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 198, 1006–1014 (2017).
Huang, S. C. C. et al. Metabolic reprogramming mediated by the mTORC2–IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45, 817–830 (2016).
Viola, A., Munari, F., Sánchez-Rodríguez, R., Scolaro, T. & Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 10, 1462 (2019).
Chen, Y. et al. Regulation of CD8+ T memory and exhaustion by the mTOR signals. Cell. Mol. Immunol. 20, 1023–1039 (2023).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Bonacina, F. et al. The low-density lipoprotein receptor–mTORC1 axis coordinates CD8+ T cell activation. J. Cell Biol. 221, e202202011 (2022).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Ma, E. H., Poffenberger, M. C., Wong, A. H. T. & Jones, R. G. The role of AMPK in T cell metabolism and function. Curr. Opin. Immunol. 46, 45–52 (2017).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Pua, H. H., Dzhagalov, I., Chuck, M., Mizushima, N. & He, Y.-W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Cell Biol. 176, 25–31 (2007).
Carleton, G. & Lum, J. J. Autophagy metabolically suppresses CD8+ T cell antitumor immunity. Autophagy 15, 1648–1649 (2019).
Wei, J. et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 17, 277–285 (2016).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Reversat, A. et al. Polarity protein Par3 controls B-cell receptor dynamics and antigen extraction at the immune synapse. Mol. Biol. Cell 26, 1273–1285 (2015).
Yuseff, M. I. et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity 35, 361–374 (2011).
Iwata, T. N. et al. Conditional disruption of raptor reveals an essential role for mTORC1 in B cell development, survival, and metabolism. J. Immunol. 197, 2250–2260 (2016).
Jia, R. & Bonifacino, J. S. Lysosome positioning influences mTORC2 and AKT signaling. Mol. Cell 75, 26–38.e3 (2019).
Marques, A. R. A. & Saftig, P. Lysosomal storage disorders — challenges, concepts and avenues for therapy: beyond rare diseases. J. Cell Sci. 132, jcs221739 (2019).
Korbelius, M., Kuentzel, K. B., Bradić, I., Vujić, N. & Kratky, D. Recent insights into lysosomal acid lipase deficiency. Trends Mol. Med. 29, 425–438 (2023). A review of the cellular, clinical and epidemiological implications of LAL-D and the consequent impaired hydrolysis of cholesterol esters and triglycerides.
Gomaraschi, M., Bonacina, F. & Norata, G. D. Lysosomal acid lipase: from cellular lipid handler to immunometabolic target. Trends Pharmacol. Sci. 40, 104–115 (2019).
Baronio, F. et al. Diagnosis, treatment, and follow-up of a case of Wolman disease with hemophagocytic lymphohistiocytosis. Mol. Genet. Metab. Rep. 30, 100833 (2022).
Qu, P., Du, H., Wilkes, D. S. & Yan, C. Critical roles of lysosomal acid lipase in T cell development and function. Am. J. Pathol. 174, 944–956 (2009).
Qu, P. et al. Critical roles of lysosomal acid lipase in myelopoiesis. Am. J. Pathol. 176, 2394–2404 (2010).
Lipiński, P. et al. Progressive macrophage accumulation in lysosomal acid lipase deficiency. Mol. Genet. Metab. Rep. 23, 100594 (2020).
Hoffman, E. P. et al. Lysosomal acid lipase deficiency. In GeneReviews (Univ. Washington, 2016).
Gomaraschi, M. et al. Lipid accumulation impairs lysosomal acid lipase activity in hepatocytes: evidence in NAFLD patients and cell cultures. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 158523 (2019).
Vanier, M. T. Niemann–Pick disease type C. Orphanet J. Rare Dis. 5, 16 (2010).
Platt, N. et al. Immune dysfunction in Niemann–Pick disease type C. J. Neurochem. 136, 74–80 (2016).
Rigante, D., Cipolla, C., Basile, U., Gulli, F. & Savastano, M. C. Overview of immune abnormalities in lysosomal storage disorders. Immunol. Lett. 188, 79–85 (2017).
DiRosario, J. et al. Innate and adaptive immune activation in the brain of MPS IIIB mouse model. J. Neurosci. Res. 87, 978–990 (2009).
Mauhin, W. et al. Innate and adaptive immune response in Fabry disease. JIMD Rep. 22, 1–10 (2015).
Jou, I. et al. Gangliosides trigger inflammatory responses via TLR4 in brain glia. Am. J. Pathol. 168, 1619–1630 (2006).
Schuchman, E. H. & Desnick, R. J. Types A and B Niemann–Pick disease. Mol. Genet. Metab. 120, 27–33 (2017).
Stirnemann, J. Ô. et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int. J. Mol. Sci. 18, 441 (2017).
Barak, V. et al. Cytokines in Gaucher’s disease. Eur. Cytokine Netw. 10, 205–210 (1999).
Shoenfeld, Y. et al. Gaucher’s disease: a disease with chronic stimulation of the immune system. Arch. Pathol. Lab. Med. 106, 388–391 (1982).
Allen, M. J., Myer, B. J., Khokher, A. M., Rushton, N. & Cox, T. M. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. QJM Int. J. Med. 90, 19–25 (1997).
Zahran, A. M. et al. Activated and memory T lymphocytes in children with Gaucher disease. Arch. Immunol. Ther. Exp. 65, 263–269 (2017).
Parenti, G., Pignata, C., Vajro, P. & Salerno, M. New strategies for the treatment of lysosomal storage diseases (review). Int. J. Mol. Med. 31, 11–20 (2013).
Klapan, K. et al. Evidence for lysosomal dysfunction within the epidermis in psoriasis and atopic dermatitis. J. Invest. Dermatol. 141, 2838–2848.e4 (2021).
Jansen, E. J. R. et al. ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat. Commun. 7, 11600 (2016).
Spalinger, M. R., Rogler, G. & Scharl, M. Crohn’s disease: loss of tolerance or a disorder of autophagy? Dig. Dis. 32, 370–377 (2014).
Blott, E. J. & Griffiths, G. M. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3, 122–131 (2002).
Griffiths, G. M. Secretory lysosomes — a special mechanism of regulated secretion in haemopoietic cells. Trends Cell Biol. 6, 329–332 (1996).
Holt, O. J., Gallo, F. & Griffiths, G. M. Regulating secretory lysosomes. J. Biochem. 140, 7–12 (2006).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Primers 5, 56 (2019).
Yvan-Charvet, L., Bonacina, F., Guinamard, R. R. & Norata, G. D. Immunometabolic function of cholesterol in cardiovascular disease and beyond. Cardiovasc. Res. 115, 1393–1407 (2019).
Bonacina, F., Da Dalt, L., Catapano, A. L. & Norata, G. D. Metabolic adaptations of cells at the vascular–immune interface during atherosclerosis. Mol. Asp. Med. 77, 100918 (2021).
Haka, A. S. et al. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell 20, 4932–4940 (2009).
Burtenshaw, D., Kitching, M., Redmond, E. M., Megson, I. L. & Cahill, P. A. Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front. Cardiovasc. Med. 6, 89 (2019).
Emanuel, R. et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 34, 1942–1952 (2014).
Perrotta, I. The use of electron microscopy for the detection of autophagy in human atherosclerosis. Micron 50, 7–13 (2013).
Chen, Y. et al. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ. Res. 125, 1087–1102 (2019).
Marques, A. R. A., Ramos, C., Machado-Oliveira, G. & Vieira, O. V. Lysosome (dys)function in atherosclerosis — a big weight on the shoulders of a small organelle. Front. Cell Dev. Biol. 9, 658995 (2021).
Ding, Z. et al. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci. Rep. 3, 1077 (2013).
Cox, B. E., Griffin, E. E., Ullery, J. C. & Jerome, W. G. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J. Lipid Res. 48, 1012–1021 (2007).
Kumar, A. et al. Role of pyruvate kinase M2 in oxidized LDL-induced macrophage foam cell formation and inflammation. J. Lipid Res. 61, 351–364 (2020).
Hajjar, S. & Zhou, X. pH sensing at the intersection of tissue homeostasis and inflammation. Trends Immunol. 44, 807–825 (2023).
Heuser, J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 108, 855–864 (1989).
Febbraio, M., Guy, E. & Silverstein, R. L. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 2333–2338 (2004).
Pfeffer, S. R. Clues to NPC1-mediated cholesterol export from lysosomes. Proc. Natl Acad. Sci. USA 113, 7941–7943 (2016).
Awan, S. et al. Wnt5a promotes lysosomal cholesterol egress and protects against atherosclerosis. Circ. Res. 130, 184–199 (2022).
Liao, X. et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 15, 545–553 (2012).
Qiao, L. et al. Deficient chaperone-mediated autophagy promotes inflammation and atherosclerosis. Circ. Res. 129, 1141–1157 (2021).
Diab, D. L. et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 6, 1249–1254 (2009).
Wen, Y. & Leake, D. S. Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ. Res. 100, 1337–1343 (2007).
Hoff, H. F. & Hoppe, G. Structure of cholesterol-containing particles accumulating in atherosclerotic lesions and the mechanisms of their derivation. Curr. Opin. Lipidol. 6, 317–325 (1995).
O’Neil, J., Hoppe, G., Sayre, L. M. & Hoff, H. F. Inactivation of cathepsin B by oxidized LDL involves complex formation induced by binding of putative reactive sites exposed at low pH to thiols on the enzyme. Free Radic. Biol. Med. 23, 215–225 (1997).
Ahmad, F. & Leake, D. S. Lysosomal oxidation of LDL alters lysosomal pH, induces senescence, and increases secretion of pro-inflammatory cytokines in human macrophages. J. Lipid Res. 60, 98–110 (2019).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Gibson, M. S., Domingues, N. & Vieira, O. V. Lipid and non-lipid factors affecting macrophage dysfunction and inflammation in atherosclerosis. Front. Physiol. 9, 654 (2018).
Wang, M. X. et al. TNF compromises lysosome acidification and reduces α-synuclein degradation via autophagy in dopaminergic cells. Exp. Neurol. 271, 112–121 (2015).
York, A. G. et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163, 1716–1729 (2015).
Canfrán-Duque, A. et al. Macrophage-derived 25-hydroxycholesterol promotes vascular inflammation, atherogenesis, and lesion remodeling. Circulation 147, 388–408 (2023).
Chu, T. T. et al. Tonic prime-boost of STING signalling mediates Niemann–Pick disease type C. Nature 596, 570–575 (2021).
Tabas, I. & Bornfeldt, K. E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118, 653–667 (2016).
Zhang, S. et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 29, 443–456.e5 (2019).
Schrijvers, D. M., De Meyer, G. R. Y., Kockx, M. M., Herman, A. G. & Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 1256–1261 (2005).
Boada-Romero, E., Martinez, J., Heckmann, B. L. & Green, D. R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 21, 398–414 (2020).
Mehrotra, P. & Ravichandran, K. S. Drugging the efferocytosis process: concepts and opportunities. Nat. Rev. Drug Discov. 21, 601–620 (2022).
Zhang, Y. et al. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 23, 898–914 (2013).
Yvan-Charvet, L. et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ. Res. 106, 1861–1869 (2010).
Li, G., Scull, C., Ozcan, L. & Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 191, 1113–1125 (2010).
Zinkevich, N. S. & Gutterman, D. D. ROS-induced ROS release in vascular biology: redox–redox signaling. Am. J. Physiol. Heart Circ. Physiol. 301, H647–H653 (2011).
Wang, Y. & Tabas, I. Emerging roles of mitochondria ROS in atherosclerotic lesions: causation or association? J. Atheroscler. Thromb. 21, 381–390 (2014).
Medina, C. B. et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 580, 130–135 (2020).
Yurdagul, A. et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 31, 518–533.e10 (2020).
Ngai, D., Schilperoort, M. & Tabas, I. Efferocytosis-induced lactate enables the proliferation of pro-resolving macrophages to mediate tissue repair. Nat. Metab. 5, 2206–2219 (2023). A study exploring the metabolic consequence of efferocytosis in the resolution of inflammation triggered by apoptotic cell digestion in the lysosome, pushing immunometabolic reprogramming of macrophages towards glycolysis and pro-resolving functions.
Curnock, R. et al. TFEB‐dependent lysosome biogenesis is required for senescence. EMBO J. 42, e111241 (2023).
Roh, K. et al. Lysosomal control of senescence and inflammation through cholesterol partitioning. Nat. Metab. 5, 398–413 (2023).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Hall, B. M. et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 9, 1867–1884 (2017).
Evangelou, K. et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16, 192–197 (2017).
De Silva, N. S. et al. Nuclear envelope disruption triggers hallmarks of aging in lung alveolar macrophages. Nat. Aging 3, 1251–1268 (2023).
Fabre, T. et al. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci. Immunol. 8, eadd8945 (2023).
Pols, M. S. & Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 315, 1584–CD1592 (2009).
Ryter, S. W., Lee, S. J., Smith, A. & Choi, A. M. K. Autophagy in vascular disease. Proc. Am. Thorac. Soc. 7, 40–47 (2010).
Grootaert, M. O. J. et al. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 11, 2014–2032 (2015).
De Meyer, G. R. Y. et al. Autophagy in vascular disease. Circ. Res. 116, 468–479 (2015).
Mandatori, S. et al. Altered Tregs differentiation and impaired autophagy correlate to atherosclerotic disease. Front. Immunol. 11, 350 (2020).
Amersfoort, J. et al. Defective autophagy in T cells impairs the development of diet-induced hepatic steatosis and atherosclerosis. Front. Immunol. 9, 2937 (2018).
Clement, M. et al. Impaired autophagy in CD11b+ dendritic cells expands CD4+ regulatory T cells and limits atherosclerosis in mice. Circ. Res. 125, 1019–1034 (2019).
Arnold, J. et al. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 23, 853–864 (2016).
Stroope, C. et al. Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities. Nat. Metab. 6, 617–638 (2024).
Bonacina, F. et al. The heterogeneous cellular landscape of atherosclerosis: implications for future research and therapies. A collaborative review from the EAS young fellows. Atherosclerosis 372, 48–56 (2023).
Schaum, N. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Schrijvers, D. M., De Meyer, G. R. Y. & Martinet, W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler. Thromb. Vasc. Biol. 31, 2787–2791 (2011).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Sergin, I. et al. Exploiting macrophage autophagy–lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 8, 15750 (2017).
Tao, H. et al. Macrophage SR-BI modulates autophagy via VPS34 complex and PPARα transcription of TFEB in atherosclerosis. J. Clin. Invest. 131, e94229 (2021).
Fang, S. et al. Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis. 12, 88 (2021).
Jeong, S. J. et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy–lysosome biogenesis response. Autophagy 17, 3740–3752 (2021).
Blessing, A. M. et al. Transcriptional regulation of core autophagy and lysosomal genes by the androgen receptor promotes prostate cancer progression. Autophagy 13, 506–521 (2017).
Carling, P. J. et al. Multiparameter phenotypic screening for endogenous TFEB and TFE3 translocation identifies novel chemical series modulating lysosome function. Autophagy 19, 692–705 (2023).
Wang, C. et al. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat. Commun. 8, 2270 (2017).
Lin, Y. et al. A small-molecule drug inhibits autophagy gene expression through the central regulator TFEB. Proc. Natl Acad. Sci. USA 120, e2213670120 (2023).
Zhang, X. et al. Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis. Autophagy 19, 886–903 (2023).
Du, H. et al. Reduction of atherosclerotic plaques by lysosomal acid lipase supplementation. Arterioscler. Thromb. Vasc. Biol. 24, 147–154 (2004).
Jia, J. et al. Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev. Cell 52, 69–87.e8 (2020).
Jia, J. et al. Galectins control mTOR in response to endomembrane damage. Mol. Cell 70, 120–135.e8 (2018).
Sharma, U. C. et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110, 3121–3128 (2004).
Falcone, C. et al. Galectin-3 plasma levels and coronary artery disease: a new possible biomarker of acute coronary syndrome. Int. J. Immunopathol. Pharmacol. 24, 905–913 (2011).
Arar, C., Gaudin, J. C., Capron, L. & Legrand, A. Galectin-3 gene (LGALS3) expression in experimental atherosclerosis and cultured smooth muscle cells. FEBS Lett. 430, 307–311 (1998).
Di Gregoli, K. et al. Galectin-3 identifies a subset of macrophages with a potential beneficial role in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 40, 1491–1509 (2020).
Shen, D. et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 3, 731 (2012).
Scotto Rosato, A. et al. TRPML1 links lysosomal calcium to autophagosome biogenesis through the activation of the CaMKKβ/VPS34 pathway. Nat. Commun. 10, 5630 (2019).
Somogyi, A. et al. The synthetic TRPML1 agonist ML-SA1 rescues Alzheimer-related alterations of the endosomal–autophagic–lysosomal system. J. Cell Sci. 136, jcs259875 (2023).
Zhong, D. et al. Induction of lysosomal exocytosis and biogenesis via TRPML1 activation for the treatment of uranium-induced nephrotoxicity. Nat. Commun. 14, 3997 (2023).
Martina, J. A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Castro, C. et al. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27Kip1-independent pathway. Atherosclerosis 172, 31–38 (2004).
Stone, G. W. et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation 124, 893–900 (2011).
Gerlach, B. D. et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 33, 2445–2463.e8 (2021).
Zhang, X. et al. Loss of macrophage mTORC2 drives atherosclerosis via FoxO1 and IL-1β signaling. Circ. Res. 133, 200–219 (2023). This research shows how elevated blood leucine levels owing to a high-protein diet promote mTORC1 activation in macrophages, increasing the risk of atherosclerosis in experimental models.
Ai, D. et al. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ. Res. 114, 1576–1584 (2014).
Zhang, Q. et al. Rheb (Ras homolog enriched in brain 1) deficiency in mature macrophages prevents atherosclerosis by repressing macrophage proliferation, inflammation, and lipid uptake. Arterioscler. Thromb. Vasc. Biol. 39, 1787–1801 (2019).
Zhang, X. et al. Identification of a leucine-mediated threshold effect governing macrophage mTOR signalling and cardiovascular risk. Nat. Metab. 6, 359–377 (2024).
Kaldirim, M. et al. Modulation of mTOR signaling in cardiovascular disease to target acute and chronic inflammation. Front. Cardiovasc. Med. 9, 907348 (2022).
Valvezan, A. J. & Manning, B. D. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab. 1, 321–333 (2019).
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013).
Shin, H. R. et al. Lysosomal GPCR-like protein LYCHOS signals cholesterol sufficiency to mTORC1. Science 377, 1290–1298 (2022). This study identifies the lysosomal cholesterol signalling protein, a G protein-coupled receptor, as a cholesterol sensor that interacts with mTORC1 and participates in its activation.
Castellano, B. M. et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9–Niemann–Pick C1 signaling complex. Science 355, 1306–1311 (2017). Report of how the transmembrane protein SLC38A9 senses LDLR-derived free cholesterol concentration in the lysosome and participates in mTORC1 clustering on the lysosomal membrane.
Menon, D. et al. ARL8B mediates lipid droplet contact and delivery to lysosomes for lipid remobilization. Cell Rep. 42, 113203 (2023).
Murley, A. et al. Ltc1 is an ER-localized sterol transporter and a component of ER–mitochondria and ER–vacuole contacts. J. Cell Biol. 209, 539–548 (2015).
Wilhelm, L. P. et al. STARD 3 mediates endoplasmic reticulum‐to‐endosome cholesterol transport at membrane contact sites. EMBO J. 36, 1412–1433 (2017).
Wu, H., Carvalho, P. & Voeltz, G. K. Here, there, and everywhere: the importance of ER membrane contact sites. Science 361, eaan5835 (2018).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Pu, J., Guardia, C. M., Keren-Kaplan, T. & Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016).
Korolchuk, V. I. et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 13, 453–462 (2011).
Mindell, J. A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86 (2012).
Acknowledgements
G.D.N. was supported by Progetti di Rilevante Interesse Nazionale (PRIN 2022 7KTSAT), Ricerca Finalizzata, Ministry of Health (RF-2019-12370896), Nanokos (European Commission Ref. EUROPEAID/173691/DD/ACT/XK), PNRR Missione 4 (Progetto CN3 — National Center for Gene Therapy and Drugs based on RNA Technology), PNRR Missione 4 (Progetto MUSA — Multilayered Urban Sustainability Action), PNRR Missione 6 (PNRR-MAD-2022-12375913) and CARDINNOV, Ministry of Research and University under the umbrella of the Partnership fostering a European Research Area for Health (ERA4Health) (GA number 101095426 of the EU Horizon Europe Research and Innovation Programme). F.B. was supported by Progetti di Rilevante Interesse Nazionale (PRIN 2022 2022NBKCWP), Fondazione Cariplo (1560-2019) and Piano di Sostegno alla Ricerca, Università degli studi di Milano (PSR2022_DIP_022_AZIONE_A_FBONA). L.Y.-C. was supported by grants from the European Research Council (ERC) consolidator programme (ERC2016COG724838), ANR (MacBurn) and IHU RespirERA (Respiratory Health, Environment and Ageing).
Author information
Authors and Affiliations
Contributions
F.B., X.Z. and N.M. researched data for the article. F.B., L.Y.-C. and G.D.N. discussed its content. F.B., X.Z., N.M., L.Y.-C. and G.D.N. wrote the manuscript. F.B., L.Y.-C., B.R. and G.D.N. reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Otilia Vieira, Hanrui Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bonacina, F., Zhang, X., Manel, N. et al. Lysosomes in the immunometabolic reprogramming of immune cells in atherosclerosis. Nat Rev Cardiol 22, 149–164 (2025). https://doi.org/10.1038/s41569-024-01072-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-024-01072-4
This article is cited by
-
Haematometabolism rewiring in atherosclerotic cardiovascular disease
Nature Reviews Cardiology (2025)
-
Obesity alters cholesterol homeostasis in regulatory T cells of visceral adipose tissue
Nature Reviews Cardiology (2025)
-
CKLF1 disrupts microglial efferocytosis following acute ischemic stroke by binding to phosphatidylserine
Cell Death & Differentiation (2025)
-
Dissecting inflammation in the immunemetabolomic era
Cellular and Molecular Life Sciences (2025)