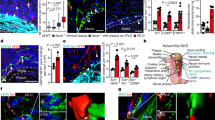
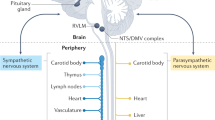
Abstract
Ischaemic heart disease is a consequence of coronary atherosclerosis, and atherosclerosis is a systemic inflammatory disease. The spleen releases various immune cells in temporally distinct patterns. Neutrophils, monocytes, macrophages, B cells and T cells execute innate and adaptive immune processes in the coronary atherosclerotic plaque and in the ischaemic myocardium. Prolonged inflammation contributes to ischaemic heart failure. The spleen is also a target of neuromodulation through vagal, sympathetic and sensory nerve activation. Efferent vagal activation and subsequent activation of the noradrenergic splenic nerve activate β2-adrenergic receptors on splenic T cells, which release acetylcholine that ultimately results in attenuation of cytokine secretion from splenic macrophages. Coeliac vagal nerve activation increases splenic sympathetic nerve activity and drives the release of T cells, a process that depends on placental growth factor. Activation of the vagosplenic axis protects acutely from ischaemia–reperfusion injury during auricular tragus vagal stimulation and remote ischaemic conditioning. Splenectomy abrogates all these deleterious and beneficial actions on the cardiovascular system. The aggregate effect of splenectomy in humans is a long-term increase in mortality from ischaemic heart disease. The spleen has been appreciated as an important immune organ for inflammatory processes in atherosclerosis, myocardial infarction and heart failure, whereas its complex interaction with circulating blood factors and with the autonomic and somatic nervous systems, as well as its role in cardioprotection, have emerged only in the past decade. In this Review, we describe this newly identified cardioprotective function of the spleen and highlight the potential for translating the findings to patients with ischaemic heart disease.
Key points
-
The spleen is a central immune organ and provides an interface to the autonomic nervous system and the circulating blood.
-
Immune cells originating in the spleen contribute to atherosclerotic plaque inflammation and the inflammatory response to myocardial infarction.
-
Efferent vagal nerve activation induces the activation of splenic noradrenergic nerves that project onto splenic T cells, which then release acetylcholine to activate α7-nicotinic receptors on macrophages, resulting in decreased macrophage cytokine release.
-
Coeliac vagal nerve activation increases splenic sympathetic nerve activity and drives the release of T cells from the spleen, a process that is mediated by placental growth factor.
-
Activation of the vagosplenic axis is decisive for myocardial infarct size reduction by auricular tragus stimulation and remote ischaemic conditioning in rats, pigs and humans.
-
Splenectomy in humans is associated with increased mortality from ischaemic heart disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heusch, G. Myocardial ischemia/reperfusion: translational pathophysiology of ischemic heart disease. Med 5, 10–31 (2024).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Döring, Y., van der Vorst, E. P. C. & Weber, C. Targeting immune cell recruitment in atherosclerosis. Nat. Rev. Cardiol. 21, 824–840 (2024).
Heusch, G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation 81, 1–13 (1990).
Heusch, G. et al. α-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101, 689–694 (2000).
Heusch, G. Vagal cardioprotection in reperfused acute myocardial infarction. JACC Cardiovasc. Interv. 10, 1521–1522 (2017).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022).
Mohanta, S. K. et al. Cardiovascular brain circuits. Circ. Res. 132, 1546–1565 (2023).
Weber, C., Habenicht, A. J. R. & von Hundelshausen, P. Novel mechanisms and therapeutic targets in atherosclerosis: inflammation and beyond. Eur. Heart J. 44, 2672–2681 (2023).
Carnevale, L. et al. Celiac vagus nerve stimulation recapitulates angiotensin II-induced splenic noradrenergic activation, driving egress of CD8 effector cells. Cell Rep. 33, 108494 (2020).
Carnevale, D. Neuroimmune axis of cardiovascular control: mechanisms and therapeutic implications. Nat. Rev. Cardiol. 19, 379–394 (2022).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
van der Laan, A. M. et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 (2014).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Rein, H. Über ein Regulationssystem “Milz-Leber” für den oxydativen Stoffwechsel der Körpergewebe und besonders des Herzens. Naturwissenschaften 36, 233–239 (1949).
Rein, H. The role of the spleen and liver in coronary or hypoxic myocardial insufficiency. Pflug. Arch. Gesamt. Physiol. Menschen Tiere 253, 435–458 (1951).
Meesmann, W. & Schmier, J. Effects of electric stimulation of the splenic nerve on coronary blood flow. Pflügers Arch. 263, 293–303 (1956).
Meesmann, W. & Schmier, J. Oxygen consumption of the heart in spleen-liver mechanism. Pflügers Arch. 263, 304–314 (1956).
Lieder, H. R. et al. Vago-splenic axis in signal transduction of remote ischemic preconditioning in pigs and rats. Circ. Res. 123, 1152–1163 (2018).
Lieder, H. et al. Vago-splenic signal transduction of cardioprotection in humans. Eur. Heart J. 45, 3164–3177 (2024).
Heusch, G. The spleen in myocardial infarction. Circ. Res. 124, 26–28 (2019).
Cesta, M. F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 34, 455–465 (2006).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Steiniger, B. S. Human spleen microanatomy: why mice do not suffice. Immunology 145, 334–346 (2015).
Alexandre, Y. O. & Mueller, S. N. Splenic stromal niches in homeostasis and immunity. Nat. Rev. Immunol. 23, 705–719 (2023).
Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci. Immunol. 4, eaau6085 (2019).
Crean, P. A. et al. The fractional distribution of the cardiac output in man using microspheres labelled with technetium 99m. Br. J. Radiol. 59, 209–215 (1986).
Steiniger, B. S., Pfeffer, H., Guthe, M. & Lobachev, O. Exploring human splenic red pulp vasculature in virtual reality: details of sheathed capillaries and the open capillary network. Histochem. Cell Biol. 155, 341–354 (2021).
Steiniger, B. S., Pfeffer, H., Gaffling, S. & Lobachev, O. The human splenic microcirculation is entirely open as shown by 3D models in virtual reality. Sci. Rep. 12, 16487 (2022).
Pereira, M. R. & Leite, P. E. The involvement of parasympathetic and sympathetic nerve in the inflammatory reflex. J. Cell Physiol. 231, 1862–1869 (2016).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Ji, H. et al. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7, 335–347 (2014).
Straub, R. H., Lang, B., Falk, W., Scholmerich, J. & Singer, E. A. In vitro superfusion method for the investigation of nerve-immune cell interaction in murine spleen. J. Neuroimmunol. 61, 53–60 (1995).
Mota, C. M. D. & Madden, C. J. Neural control of the spleen as an effector of immune responses to inflammation: mechanisms and treatments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 323, R375–R384 (2022).
Gonzalez-Gonzalez, M. A., Bendale, G. S., Wang, K., Wallace, G. G. & Romero-Ortega, M. Platinized graphene fiber electrodes uncover direct spleen-vagus communication. Commun. Biol. 4, 1097 (2021).
Kawashima, K., Fujii, T., Moriwaki, Y., Misawa, H. & Horiguchi, K. Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs. Int. Immunopharmacol. 29, 127–134 (2015).
Inoue, T. et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Invest. 126, 1939–1952 (2016).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Bratton, B. O. et al. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol. 97, 1180–1185 (2012).
Kobori, N., Moore, A. N., Redell, J. B. & Dash, P. K. Caudal DMN neurons innervate the spleen and release CART peptide to regulate neuroimmune function. J. Neuroinflammation 20, 158 (2023).
Tanaka, S. et al. Vagus nerve stimulation activates two distinct neuroimmune circuits converging in the spleen to protect mice from kidney injury. Proc. Natl Acad. Sci. USA 118, e2021758118 (2021).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Zschaler, J., Schlorke, D. & Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 34, 433–454 (2014).
Donega, M. et al. Human-relevant near-organ neuromodulation of the immune system via the splenic nerve. Proc. Natl Acad. Sci. USA 118, e2025428118 (2021).
Verlinden, T. J. M. et al. Innervation of the human spleen: a complete hilum-embedding approach. Brain Behav. Immun. 77, 92–100 (2019).
Wu, M. et al. Innervation of nociceptor neurons in the spleen promotes germinal center responses and humoral immunity. Cell 187, 2935–2951 (2024).
Medeiros, A., Peres-Buzalaf, C., Fortino Verdan, F. & Serezani, C. H. Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediators Inflamm. 2012, 327568 (2012).
Gilmore, N., Vane, J. R. & Wyllie, J. H. Prostaglandins released by the spleen. Nature 218, 1135–1140 (1968).
Smith, J. N. et al. 15-PGDH inhibition activates the splenic niche to promote hematopoietic regeneration. JCI Insight 6, e143658 (2021).
Wolfrum, S. et al. Calcitonin gene related peptide mediates cardioprotection by remote preconditioning. Regul. Pept. 127, 217–224 (2005).
Ketelhuth, D. F. & Hansson, G. K. Adaptive response of T and B cells in atherosclerosis. Circ. Res. 118, 668–678 (2016).
Wagner, J. U. G. et al. Aging impairs the neurovascular interface in the heart. Science 381, 897–906 (2023).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 10, 4138–4149 (2011).
Westerterp, M. et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206 (2012).
Al-Sharea, A. et al. Nicotinic acetylcholine receptor alpha 7 stimulation dampens splenic myelopoiesis and inhibits atherogenesis in Apoe−/− mice. Atherosclerosis 265, 47–53 (2017).
Potteaux, S., Ait-Oufella, H. & Mallat, Z. Role of splenic monocytes in atherosclerosis. Curr. Opin. Lipidol. 26, 457–463 (2015).
Fernandez-Garcia, V., Gonzalez-Ramos, S., Martin-Sanz, P., Castrillo, A. & Bosca, L. Contribution of extramedullary hematopoiesis to atherosclerosis. The spleen as a neglected hub of inflammatory cells. Front. Immunol. 11, 586527 (2020).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Yan, C., Li, Y. Z., Luo, X. M., Quan, X. J. & Feng, Y. M. Roles of hematopoietic stem and progenitor cells in ischemic cardiovascular disease. Curr. Stem Cell Res. Ther. 16, 589–598 (2021).
Asai, K., Kuzuya, M., Naito, M., Funaki, C. & Kuzuya, F. Effects of splenectomy on serum lipids and experimental atherosclerosis. Angiology 39, 497–504 (1988).
Ai, X. M. et al. The role of splenectomy in lipid metabolism and atherosclerosis (AS). Lipids Health Dis. 17, 186 (2018).
Akbar, N. et al. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight 2, e93344 (2017).
Akbar, N. et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc. Res. 119, 236–251 (2023).
Panda, R. & Kubes, P. Extracellular vesicles selectively mobilize splenic neutrophils. Cardiovasc. Res. 119, 1–2 (2023).
Rasheed, A. et al. Hyperlipidemia-induced hematopoiesis is repressed by MLKL in endothelial cells of the splenic niche. Nat. Cardiovasc. Res. 3, 594–611 (2024).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Soehnlein, O. & Libby, P. Targeting inflammation in atherosclerosis – from experimental insights to the clinic. Nat. Rev. Drug. Discov. 20, 589–610 (2021).
Tay, C. et al. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc. Res. 111, 385–397 (2016).
Grasset, E. K. et al. Sterile inflammation in the spleen during atherosclerosis provides oxidation-specific epitopes that induce a protective B-cell response. Proc. Natl Acad. Sci. USA 112, E2030–E2038 (2015).
Jones, P. W., Mallat, Z. & Nus, M. T-cell/B-cell interactions in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 44, 1502–1511 (2024).
O’Brien, J. W., Case, A., Kemper, C., Zhao, T. X. & Mallat, Z. Therapeutic avenues to modulate B-cell function in patients with cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 44, 1512–1522 (2024).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Tarnawski, L. et al. Cholinergic regulation of vascular endothelial function by human ChAT+ T cells. Proc. Natl Acad. Sci. USA 120, e2212476120 (2023).
Wang, Z. et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat. Cardiovasc. Res. 2, 290–306 (2023).
Khan, A., Roy, P. & Ley, K. Breaking tolerance: the autoimmune aspect of atherosclerosis. Nat. Rev. Immunol. 24, 670–679 (2024).
Dutta, P. et al. E-selectin inhibition mitigates splenic HSC activation and myelopoiesis in hypercholesterolemic mice with myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 36, 1802–1808 (2016).
Natarajan, N. & Dutta, P. ‘Training’ of innate immunity following myocardial infarction exacerbates atherosclerosis. Eur. Heart J. 45, 685–687 (2024).
Dong, Z. et al. Myocardial infarction drives trained immunity of monocytes, accelerating atherosclerosis. Eur. Heart J. 45, 669–684 (2024).
Riksen, N. P., Bekkering, S., Mulder, W. J. M. & Netea, M. G. Trained immunity in atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 20, 799–811 (2023).
Tian, Y. et al. The spleen contributes importantly to myocardial infarct exacerbation during post-ischemic reperfusion in mice via signaling between cardiac HMGB1 and splenic RAGE. Basic. Res. Cardiol. 111, 62 (2016).
Hilgendorf, I., Frantz, S. & Frangogiannis, N. G. Repair of the infarcted heart: cellular effectors, molecular mechanisms and therapeutic opportunities. Circ. Res. 134, 1718–1751 (2024).
Ramos-Regalado, L., Alcover, S., Badimon, L. & Vilahur, G. The influence of metabolic risk factors on the inflammatory response triggered by myocardial infarction: bridging pathophysiology to treatment. Cells 13, 1125 (2024).
Xie, D. et al. Splenic monocytes mediate inflammatory response and exacerbate myocardial ischemia/reperfusion injury in a mitochondrial cell-free DNA-TLR9-NLRP3-dependent fashion. Basic. Res. Cardiol. 118, 44 (2023).
Yap, J. et al. Macrophages in cardiac remodelling after myocardial infarction. Nat. Rev. Cardiol. 20, 373–385 (2023).
Dewald, O. et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 (2005).
Dutta, P. et al. Myocardial infarction activates CCR2+ hematopoietic stem and progenitor cells. Cell Stem Cell 16, 477–487 (2015).
Heusch, G., Deussen, A. & Thämer, V. Cardiac sympathetic nerve activity and progressive vasoconstriction distal to coronary stenoses: feed-back aggravation of myocardial ischemia. J. Auton. Nerv. Syst. 13, 311–326 (1985).
Swirski, F. K. & Nahrendorf, M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744 (2018).
Moggio, A., Schunkert, H., Kessler, T. & Sager, H. B. Quo vadis? Immunodynamics of myeloid cells after myocardial infarction. Int. J. Mol. Sci. 23, 15814 (2022).
Dewald, O. et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 164, 665–677 (2004).
Yan, X. et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell Cardiol. 62, 24–35 (2013).
Lindsey, M. L. et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 314, H812–H838 (2018).
Schnitter, F. et al. Characterizing the immune response to myocardial infarction in pigs. Basic. Res. Cardiol. 119, 453–479 (2024).
Weinberger, T. et al. Resident and recruited macrophages differentially contribute to cardiac healing after myocardial ischemia. eLife 12, RP89377 (2024).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Heinrichs, M. et al. The healing myocardium mobilizes a distinct B-cell subset through a CXCL13-CXCR5-dependent mechanism. Cardiovasc. Res. 117, 2664–2676 (2021).
Rieckmann, M. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J. Clin. Invest. 129, 4922–4936 (2019).
Delgobo, M. et al. Myocardial milieu favors local differentiation of regulatory T cells. Circ. Res. 132, 565–582 (2023).
Gladow, N. et al. Role of CD4+ T-cells for regulating splenic myelopoiesis and monocyte differentiation after experimental myocardial infarction. Basic. Res. Cardiol. 119, 261–275 (2024).
Liu, H. et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4+ T lymphocytes after myocardial infarction. J. Mol. Cell Cardiol. 91, 123–133 (2016).
Wu, L. et al. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc. Natl Acad. Sci. USA 116, 21673–21684 (2019).
Zouggari, Y. et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19, 1273–1280 (2013).
Sun, Y. et al. Splenic marginal zone B lymphocytes regulate cardiac remodeling after acute myocardial infarction in mice. J. Am. Coll. Cardiol. 79, 632–647 (2022).
Fredman, G. & Serhan, C. N. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 21, 808–823 (2024).
Halade, G. V., Norris, P. C., Kain, V., Serhan, C. N. & Ingle, K. A. Splenic leukocytes define the resolution of inflammation in heart failure. Sci. Signal. 11, eaao1818 (2018).
Gao, X. M. et al. Splenic release of platelets contributes to increased circulating platelet size and inflammation after myocardial infarction. Clin. Sci. 130, 1089–1104 (2016).
Lieder, H. R. et al. Platelet-mediated transfer of cardioprotection by remote ischemic conditioning and its abrogation by aspirin, but not by ticagrelor. Cardiovasc. Drugs Ther. 37, 865–876 (2023).
Kleinbongard, P., Andreadou, I. & Vilahur, G. The platelet paradox of injury versus protection in myocardial infarction – has it been overlooked? Basic. Res. Cardiol. 116, 37 (2021).
Tomczyk, M. et al. Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice. Basic. Res. Cardiol. 112, 39 (2017).
Ismahil, M. A. et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 114, 266–282 (2014).
Prabhu, S. D. The cardiosplenic axis is essential for the pathogenesis of ischemic heart failure. Trans. Am. Clin. Climatol. Assoc. 129, 202–214 (2018).
Bryan, A. M. & Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 20, e12836 (2018).
Means, C. K. & Brown, J. H. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200 (2009).
Keul, P. et al. Sphingosine-1-phosphate receptor 1 regulates cardiac function by modulating Ca2+ sensitivity and Na+/H+ exchange and mediates protection by ischemic preconditioning. J. Am. Heart Assoc. 5, e003393 (2016).
Gowda, S. B. et al. Sphingosine-1-phosphate interactions in the spleen and heart reflect extent of cardiac repair in mice and failing human hearts. Am. J. Physiol. Heart Circ. Physiol. 321, H599–H611 (2021).
Antipenko, S. et al. Neutrophils are indispensable for adverse cardiac remodeling in heart failure. J. Mol. Cell Cardiol. 189, 1–11 (2024).
Bansal, S. S. et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ. Heart Fail. 10, e003688 (2017).
Adamo, L., Rocha-Resende, C., Prabhu, S. D. & Mann, D. L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 17, 269–285 (2020).
Maeda, D. et al. Splenic volume index determined using computed tomography upon admission is associated with readmission for heart failure among patients with acute decompensated heart failure. Int. Heart J. 62, 584–591 (2021).
Hiraiwa, H. et al. Splenic size as an indicator of hemodynamics and prognosis in patients with heart failure. Heart Vessel. 37, 1344–1355 (2022).
Maisel, A. et al. Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circ. Res. 82, 458–463 (1998).
Adamo, L. et al. B cell-mediated antigen presentation promotes adverse cardiac remodeling in chronic heart failure. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-4536350/v1 (2024).
Kelly, M. J., Breathnach, C., Tracey, K. J. & Donnelly, S. C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 3, 100696 (2022).
Kaplan, A. et al. Cooling down inflammation in the cardiovascular system via the nicotinic acetylcholine receptor. J. Cardiovasc. Pharmacol. 82, 241–265 (2023).
Fang, J. et al. α7nAChR deletion aggravates myocardial infarction and enhances systemic inflammatory reaction via mTOR-signaling-related autophagy. Inflammation 42, 1190–1202 (2019).
Yu, L. et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc. Interv. 10, 1511–1520 (2017).
Heusch, G., Bøtker, H. E., Przyklenk, K., Redington, A. & Yellon, D. M. Remote ischemic conditioning. J. Am. Coll. Cardiol. 65, 177–195 (2015).
Hausenloy, D. J. et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet 370, 575–579 (2007).
Thielmann, M. et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382, 597–604 (2013).
Kleinbongard, P., Peters, J., Jakob, H., Heusch, G. & Thielmann, M. Persistent survival benefit from remote ischemic preconditioning in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 71, 251–262 (2018).
Bøtker, H. E. et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375, 727–734 (2010).
Gaspar, A. et al. Randomized controlled trial of remote ischemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic. Res. Cardiol. 113, 14 (2018).
Hildebrandt, H. A. et al. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic. Transl. Sci. 1, 3–13 (2016).
Skyschally, A. et al. Humoral transfer and intra-myocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am. J. Physiol. Heart Circ. Physiol. 315, H159–H172 (2018).
Badimon, J., Kiss, A. & Podesser, B. K. Spleen in action for cardioprotection. Eur. Heart J. 45, 3178–3180 (2024).
Tian, Y. et al. Stimulation of the beta2 adrenergic receptor at reperfusion limits myocardial reperfusion injury via an interleukin-10-dependent anti-inflammatory pathway in the spleen. Circ. J. 82, 2829–2836 (2018).
Pang, L. X. et al. Bone marrow-derived mesenchymal stem cells attenuate myocardial ischemia-reperfusion injury via upregulation of splenic regulatory T cells. BMC Cardiovasc. Disord. 21, 215 (2021).
Garnica, M. R., Silva, J. S. & de Andrade Junior, H. F. Stromal cell-derived factor-1 production by spleen cells is affected by nitric oxide in protective immunity against blood-stage Plasmodium chabaudi CR in C57BL/6j mice. Immunol. Lett. 89, 133–142 (2003).
Davidson, S. M. et al. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic. Res. Cardiol. 108, 377 (2013).
Korf-Klingebiel, M. et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 21, 140–149 (2015).
Ruthirago, D., Julayanont, P., Tantrachoti, P., Kim, J. & Nugent, K. Cardiac arrhythmias and abnormal electrocardiograms after acute stroke. Am. J. Med. Sci. 351, 112–118 (2016).
Scheitz, J. F., Stengl, H., Nolte, C. H., Landmesser, U. & Endres, M. Neurological update: use of cardiac troponin in patients with stroke. J. Neurol. 268, 2284–2292 (2021).
Mochmann, H. C. et al. Coronary angiographic findings in acute ischemic stroke patients with elevated cardiac troponin: the TRoponin ELevation in Acute Ischemic Stroke (TRELAS) Study. Circulation 133, 1228–1229 (2016).
Blaszczyk, E. et al. Myocardial injury in patients with acute ischemic stroke detected by cardiovascular magnetic resonance imaging. Eur. J. Radiol. 165, 110908 (2023).
Stengl, H. et al. Frequency, associated variables, and outcomes of acute myocardial injury according to the fourth universal definition of myocardial infarction in patients with acute ischemic stroke. Eur. Stroke J. 7, 413–420 (2022).
Bourhy, L. et al. Neuro-inflammatory response and brain-peripheral crosstalk in sepsis and stroke. Front. Immunol. 13, 834649 (2022).
Cao, J. et al. DNA-sensing inflammasomes cause recurrent atherosclerotic stroke. Nature 633, 433–441 (2024).
Courties, G., Moskowitz, M. A. & Nahrendorf, M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 71, 233–236 (2014).
Ding, Y., DeGracia, D., Geng, X. & Ding, Y. Perspectives on effect of spleen in ischemic stroke. Brain Circ. 8, 117–120 (2022).
Ran, Y. et al. Splenectomy fails to provide long-term protection against ischemic stroke. Aging Dis. 9, 467–479 (2018).
Sternak, M., Glasnovic, A., Josic, P., Romic, D. & Gajovic, S. The effects of splenectomy in murine models of ischemic stroke: a systematic review and meta-analysis. J. Neuroinflammation 19, 233 (2022).
Han, D., Liu, H., Gao, Y. & Feng, J. Targeting brain-spleen crosstalk after stroke: new insights into stroke pathology and treatment. Curr. Neuropharmacol. 19, 1590–1605 (2021).
Yu, H. et al. The “dialogue” between central and peripheral immunity after ischemic stroke: focus on spleen. Front. Immunol. 12, 792522 (2021).
Simats, A. et al. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell 187, 4637–4655.e26 (2024).
Liu, C., Yang, J., Zhang, C., Geng, X. & Zhao, H. The changes of systemic immune responses during the neuroprotection induced by remote ischemic postconditioning against focal cerebral ischemia in mice. Neurol. Res. 41, 26–36 (2019).
Yu, H. H. et al. Remote limb ischemic postconditioning protects against ischemic stroke by promoting regulatory T cells thriving. J. Am. Heart Assoc. 10, e023077 (2021).
Chen, C. et al. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J. Neuroinflammation 15, 167 (2018).
Kees, M. G., Pongratz, G., Kees, F., Schölmerich, J. & Straub, R. H. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J. Neuroimmunol. 145, 77–85 (2003).
Rogausch, H., del Rey, A., Oertel, J. & Besedovsky, H. O. Norepinephrine stimulates lymphoid cell mobilization from the perfused rat spleen via β-adrenergic receptors. Am. J. Physiol. 276, R724–R730 (1999).
Grisanti, L. A. et al. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 134, 153–167 (2016).
Grisanti, L. A. et al. Prior β-blocker treatment decreases leukocyte responsiveness to injury. JCI Insight 5, e99485 (2019).
Leuschner, F. et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 107, 1364–1373 (2010).
Mao, Y. et al. Nanoparticle-mediated delivery of pitavastatin to monocytes/macrophages inhibits left ventricular remodeling after acute myocardial infarction by inhibiting monocyte-mediated inflammation. Int. Heart J. 58, 615–623 (2017).
Ferreira, S. H., Moncada, S. & Vane, J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat. New Biol. 231, 237–239 (1971).
Zhang, X. et al. Aspirin attenuates cardiac allograft rejection by inhibiting the maturation of dendritic cells via the NF-κB signaling pathway. Front. Pharmacol. 12, 706748 (2021).
Huang, Z. et al. Ticagrelor regulates the differentiation of MDSCs after acute myocardial infarction to reduce cardiac injury. Biomed. Pharmacother. 172, 116209 (2024).
Kottenberg, E. et al. Protection by remote ischaemic preconditioning during coronary artery bypass grafting with isoflurane but not with propofol anesthesia – a clinical trial. Acta Anaesthesiol. Scand. 56, 30–38 (2012).
Ruparelia, N. et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur. Heart J. 36, 1923–1934 (2015).
Scheitz, J. F. et al. Stroke-heart syndrome: recent advances and challenges. J. Am. Heart Assoc. 11, e026528 (2022).
Emami, H. et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 8, 121–130 (2015).
Patel, N. H. et al. Heightened splenic and bone marrow uptake of 18F-FDG PET/CT is associated with systemic inflammation and subclinical atherosclerosis by CCTA in psoriasis: an observational study. Atherosclerosis 339, 20–26 (2021).
Rorholt, M., Ghanima, W., Farkas, D. K. & Norgaard, M. Risk of cardiovascular events and pulmonary hypertension following splenectomy – a Danish population-based cohort study from 1996-2012. Haematologica 102, 1333–1341 (2017).
Robinette, C. D. & Fraumeni, J. F. Jr Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet 2, 127–129 (1977).
Kristinsson, S. Y., Gridley, G., Hoover, R. N., Check, D. & Landgren, O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica 99, 392–398 (2014).
Long, B., Koyfman, A. & Gottlieb, M. Complications in the adult asplenic patient: a review for the emergency clinician. Am. J. Emerg. Med. 44, 452–457 (2021).
Palmer, J. A., Rosenthal, N., Teichmann, S. A. & Litvinukova, M. Revisiting cardiac biology in the era of single cell and spatial omics. Circ. Res. 134, 1681–1702 (2024).
Mocci, G. et al. Single-cell gene-regulatory networks of advanced symptomatic atherosclerosis. Circ. Res. 134, 1405–1423 (2024).
Bajpai, G. et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 24, 1234–1245 (2018).
de Winter, N. et al. Persistent transcriptional changes in cardiac adaptive immune cells following myocardial infarction: new evidence from the re-analysis of publicly available single cell and nuclei RNA-sequencing data sets. J. Mol. Cell Cardiol. 192, 48–64 (2024).
Acknowledgements
This article is dedicated to the memory of G.H.’s predecessor as chair of the Institute for Pathophysiology at the University of Essen Medical School, Werner Meesmann, who had been a scholar of Hermann Rein. We appreciate the assistance of E. A. Chowanietz in the collection of references and preliminary drafts for the figures. G.H. and P.K. were supported by the German Research Foundation (CRC 1116 B8, RTG 2989 P5), the European Union Cost Action CARDIOPROTECTION (CA 16225 and IGI 16225) and METAHEART (CA22169).
Author information
Authors and Affiliations
Contributions
G.H. wrote the manuscript. Both authors researched data for the article, discussed its content, and reviewed or edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Daniela Carnevale, Andrew Murphy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heusch, G., Kleinbongard, P. The spleen in ischaemic heart disease. Nat Rev Cardiol 22, 497–509 (2025). https://doi.org/10.1038/s41569-024-01114-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-024-01114-x
This article is cited by
-
Cardiac lymphatics: functional plasticity in development, disease, and precision-targeted therapies
Basic Research in Cardiology (2026)
-
Pathological mechanisms and treatment strategies for immune checkpoint inhibitor-associated myocarditis: insights from single-cell sequencing
Basic Research in Cardiology (2026)
-
Immunometabolism in heart failure
Nature Reviews Cardiology (2025)
-
Systemic downregulation of EV-associated MiRNAs following remote ischemic preconditioning
Scientific Reports (2025)
-
The cGAS-STING pathway promotes acute ischemia-induced neutropoiesis and neutrophil priming in the bone marrow
Basic Research in Cardiology (2025)