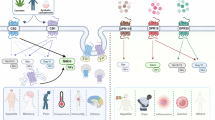
Abstract
Cannabis has been consumed for centuries, but global regulatory changes over the past three decades have increased the availability and consumption of cannabis. Cannabinoids are touted to have therapeutic potential for many diseases and could be a replacement for opioids for analgesia and sedation. However, cannabinoids can cause substantial adverse cardiovascular events that would mitigate any potential benefit. The endocannabinoid system regulates mood, satiety and memory, and modulates the cardiovascular system. The link between cannabinoids and cardiovascular disease, which used to be limited to evidence from preclinical studies, case reports and case series, is now evident in epidemiological studies. Cannabinoids adversely affect the cardiovascular system, causing myocardial infarction, cerebrovascular accidents, arrhythmia and heart failure. The effects of novel cannabinoids are unknown, and synthetic cannabinoids have the potential to cause even more substantial harm than traditional cannabinoids. Therefore, with the increasing availability and use of cannabis, the acute and chronic effects of this drug are becoming apparent.
Key points
-
Cannabis use has increased as a result of decriminalization and legalization, but the cardiovascular effects need research to inform public health policies.
-
Cannabis, via cannabinoid receptor 1 (CB1)-mediated oxidative stress and inflammation, is linked to adverse cardiovascular outcomes, including myocardial infarction, arrhythmias and cardiomyopathy.
-
CB1 antagonists and CB2 agonists are promising novel treatments for cardiovascular risk factors and cardiovascular disease, but clinical translation is complicated by adverse effects and limited data.
-
Synthetic cannabinoids (such as ‘K2’ and ‘Spice’) are an emerging public health concern owing to their potent toxicity and cardiovascular implications.
-
The co-use of cannabis and tobacco has synergistic adverse effects on cardiovascular health and addiction potential.
-
Induced pluripotent stem cell modelling and genetic tools should be used to discover novel cannabinoid signalling pathways and potential new therapeutic targets for cardiometabolic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Page, R. L. et al. Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation 142, e131–e152 (2020).
Chandy, M. et al. Adverse impact of cannabis on human health. Annu. Rev. Med. 75, 353–367 (2024).
Bridgeman, M. B. & Abazia, D. T. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T 42, 180–188 (2017).
Ryan, J. E., McCabe, S. E. & Boyd, C. J. Medicinal cannabis: policy, patients, and providers. Policy Polit. Nurs. Pract. 22, 126–133 (2021).
Hoffman, A. F. & Lupica, C. R. Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb. Perspect. Med. 3, a012237 (2013).
Krishna Kumar, K. et al. Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 176, 448–458.e12 (2019).
Sugamura, K. et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 119, 28–36 (2009).
Netherland, C. D., Pickle, T. G., Bales, A. & Thewke, D. P. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis 213, 102–108 (2010).
Hoyer, F. F. et al. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J. Mol. Cell Cardiol. 51, 1007–1014 (2011).
Pacher, P., Steffens, S., Haskó, G., Schindler, T. H. & Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat. Rev. Cardiol. 15, 151–166 (2018).
Whiting, P. F. et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313, 2456–2473 (2015).
Bilbao, A. & Spanagel, R. Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 20, 259 (2022).
Bicket, M. C., Stone, E. M. & McGinty, E. E. Use of cannabis and other pain treatments among adults with chronic pain in US states with medical cannabis programs. JAMA Netw. Open. 6, e2249797 (2023).
Zehra, A. et al. Cannabis addiction and the brain: a review. J. Neuroimmune Pharmacol. 13, 438–452 (2018).
Secades-Villa, R., Garcia-Rodriguez, O., Jin, C. J., Wang, S. & Blanco, C. Probability and predictors of the cannabis gateway effect: a national study. Int. J. Drug. Policy 26, 135–142 (2015).
Volkow, N. D., Baler, R. D., Compton, W. M. & Weiss, S. R. Adverse health effects of marijuana use. N. Engl. J. Med. 370, 2219–2227 (2014).
Wei, T. T. et al. Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell 185, 1676–1693.e23 (2022).
DeFilippis, E. M. et al. Cocaine and marijuana use among young adults with myocardial infarction. J. Am. Coll. Cardiol. 71, 2540–2551 (2018).
Patton, D. V. A history of United States cannabis law. J. Law Health 34, 1–29 (2020).
Hall, W. et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 394, 1580–1590 (2019).
Mead, A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front. Plant. Sci. 10, 697 (2019).
Lampe, J. R. Legal consequences of rescheduling marijuana. Congressional Research Service crsreports.congress.gov/product/pdf/LSB/LSB11105 (2024).
Shirah, B. H., Ahmed, M. M. & Saleh, R. A. in Medicinal Usage of Cannabis and Cannabinoids (eds Preedy, V. R., Patel, V. B. & Martin, C. R.) Ch. 5, 51–61 (Academic Press, 2023).
Bahji, A. & Stephenson, C. International perspectives on the implications of cannabis legalization: a systematic review & thematic analysis. Int. J. Env. Res. Public. Health 16, 3095 (2019).
Rotermann, M. What has changed since cannabis was legalized? Health Rep. 31, 11–20 (2020).
Martins, S. S. et al. Racial and ethnic differences in cannabis use following legalization in US states with medical cannabis laws. JAMA Netw. Open. 4, e2127002 (2021).
Chemtob, D. Forbes Daily: The Budding $28B Hemp Market’s Feud with Marijuana. Forbes www.forbes.com/sites/daniellechemtob/2024/04/19/forbes-daily-the-budding-28b-hemp-markets-feud-with-marijuana/ (2024).
Fischer, B., Jutras-Aswad, D. & Hall, W. Outcomes associated with nonmedical cannabis legalization policy in Canada: taking stock at the 5-year mark. CMAJ 195, E1351–E1353 (2023).
Chong, W. W., Acar, Z. I., West, M. L. & Wong, F. A scoping review on the medical and recreational use of cannabis during the COVID-19 pandemic. Cannabis Cannabinoid Res. 7, 591–602 (2022).
Mackie, K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 46, 101–122 (2006).
Movahed, P. et al. Vascular effects of anandamide and N-acylvanillylamines in the human forearm and skin microcirculation. Br. J. Pharmacol. 146, 171–179 (2005).
Pacher, P., Batkai, S. & Kunos, G. Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 599-625 (2005).
Pacher, P., Batkai, S. & Kunos, G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology 48, 1130–1138 (2005).
Batkai, S. et al. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am. J. Physiol. Heart Circ. Physiol 293, H1689–H1695 (2007).
Valenta, I. et al. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: a translational approach. JACC Cardiovasc. Imaging 11, 320–332 (2018).
Rajesh, M. et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61, 716–727 (2012).
Mukhopadhyay, P. et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 50, 528–536 (2007).
Mukhopadhyay, P. et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 85, 773–784 (2010).
Rajesh, M. et al. Cannabinoid receptor 2 activation alleviates diabetes-induced cardiac dysfunction, inflammation, oxidative stress, and fibrosis. Geroscience 44, 1727–1741 (2022).
Matyas, C. et al. Interplay of liver–heart inflammatory axis and cannabinoid 2 receptor signaling in an experimental model of hepatic cardiomyopathy. Hepatology 71, 1391–1407 (2020).
Molica, F. et al. Cannabinoid receptor CB2 protects against balloon-induced neointima formation. Am. J. Physiol. Heart Circ. Physiol 302, H1064–H1074 (2012).
Koivisto, A. P., Belvisi, M. G., Gaudet, R. & Szallasi, A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug. Discov. 21, 41–59 (2022).
Pertwee, R. G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 17, 1360–1381 (2010).
Després, J. P., Golay, A. & Sjöström, L. Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 353, 2121–2134 (2005).
Onakpoya, I. J., Heneghan, C. J. & Aronson, J. K. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. Crit. Rev. Toxicol. 46, 477–489 (2016).
Cinar, R., Iyer, M. R. & Kunos, G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol. Ther. 208, 107477 (2020).
Crater, G. D., Lalonde, K., Ravenelle, F., Harvey, M. & Despres, J. P. Effects of CB1R inverse agonist, INV-202, in patients with features of metabolic syndrome. A randomized, placebo-controlled, double-blind phase 1b study. Diabetes Obes. Metab. 26, 642–649 (2024).
Hashiesh, H. M., Sharma, C., Goyal, S. N., Jha, N. K. & Ojha, S. Pharmacological properties, therapeutic potential and molecular mechanisms of JWH133, a CB2 receptor-selective agonist. Front. Pharmacol. 12, 702675 (2021).
Kosar, M. et al. Patent review of cannabinoid receptor type 2 (CB2R) modulators (2016–present). Expert. Opin. Ther. Pat. 34, 665–700 (2024).
Smoum, R. et al. Editorial: therapeutic potential of the cannabinoid CB2 receptor. Front. Pharmacol. 13, 1039564 (2022).
Morales, P., Hernandez-Folgado, L., Goya, P. & Jagerovic, N. Cannabinoid receptor 2 (CB2) agonists and antagonists: a patent update. Expert. Opin. Ther. Pat. 26, 843–856 (2016).
Oparil, S. et al. Hypertension. Nat. Rev. Dis. Primers 4, 18014 (2018).
Alshaarawy, O. & Elbaz, H. A. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005–2012. J. Hypertens. 34, 1507–1512 (2016).
Golosova, D., Levchenko, V., Kravtsova, O., Palygin, O. & Staruschenko, A. Acute and long-term effects of cannabinoids on hypertension and kidney injury. Sci. Rep. 12, 6080 (2022).
Sultan, S. R., Millar, S. A., O’Sullivan, S. E. & England, T. J. A systematic review and meta-analysis of the in vivo haemodynamic effects of Δ8-tetrahydrocannabinol. Pharmaceuticals 11, 13 (2018).
Intengan, H. D. & Schiffrin, E. L. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 36, 312–318 (2000).
Corroon, J., Grant, I., Bradley, R. & Allison, M. A. Trends in cannabis use, blood pressure, and hypertension in middle-aged adults: findings from NHANES, 2009–2018. Am. J. Hypertens. 36, 651–659 (2023).
Vallee, A. Association between cannabis use and blood pressure levels according to comorbidities and socioeconomic status. Sci. Rep. 13, 2069 (2023).
Batkai, S. et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 110, 1996–2002 (2004).
Godlewski, G. et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem. Biol. 17, 1256–1266 (2010).
Wagner, J. A. et al. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature 390, 518–521 (1997).
Varga, K., Wagner, J. A., Bridgen, D. T. & Kunos, G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 12, 1035–1044 (1998).
Batkai, S. et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 7, 827–832 (2001).
Ros, J. et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology 122, 85–93 (2002).
Shan, R. et al. Activation of cannabinoid type 2 receptor in microglia reduces neuroinflammation through inhibiting aerobic glycolysis to relieve hypertension. Biomolecules 14, 333 (2024).
Guo, Y. et al. Cannabidiol protects against acute aortic dissection by inhibiting macrophage infiltration and PMAIP1-induced vascular smooth muscle cell apoptosis. J. Mol. Cell Cardiol. 189, 38–51 (2024).
Curioni, C. & Andre, C. Rimonabant for overweight or obesity. Cochrane Database Syst. Rev. 2006, CD006162 (2006).
Vidot, D. C. et al. Metabolic syndrome among marijuana users in the United States: an analysis of National Health and Nutrition Examination Survey data. Am. J. Med. 129, 173–179 (2016).
Mousavi, S. E., Tondro Anamag, F. & Sanaie, S. Association between cannabis use and risk of diabetes mellitus type 2: a systematic review and meta-analysis. Phytother. Res. 37, 5092–5108 (2023).
Ravi, D., Ghasemiesfe, M., Korenstein, D., Cascino, T. & Keyhani, S. Associations between marijuana use and cardiovascular risk factors and outcomes: a systematic review. Ann. Intern. Med. 168, 187–194 (2018).
Despres, J. P. et al. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 29, 416–423 (2009).
Muniyappa, R. et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 36, 2415–2422 (2013).
Casier, I., Vanduynhoven, P., Haine, S., Vrints, C. & Jorens, P. G. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta Cardiol. 69, 131–136 (2014).
Jeffers, A. M., Glantz, S., Byers, A. L. & Keyhani, S. Association of cannabis use with cardiovascular outcomes among US adults. J. Am. Heart Assoc. 13, e030178 (2024).
Frost, L., Mostofsky, E., Rosenbloom, J. I., Mukamal, K. J. & Mittleman, M. A. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am. Heart J. 165, 170–175 (2013).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–8743 (2002).
Rajesh, M. et al. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 160, 688–700 (2010).
El-Remessy, A. B. et al. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia 54, 1567–1578 (2011).
Paik, D. T., Chandy, M. & Wu, J. C. Patient and disease-specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol. Rev. 72, 320–342 (2020).
Yang, Z., Kulkarni, K., Zhu, W. & Hu, M. Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer. Agents Med. Chem. 12, 1264–1280 (2012).
Dol-Gleizes, F. et al. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 29, 12–18 (2009).
Steffens, S. et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434, 782–786 (2005).
Perez-Reyes, M., Owens, S. M. & Di Guiseppi, S. The clinical pharmacology and dynamics of marihuana cigarette smoking. J. Clin. Pharmacol. 21, 201S–207S (1981).
Huestis, M. A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 4, 1770–1804 (2007).
Holt, A. et al. Cannabis for chronic pain: cardiovascular safety in a nationwide Danish study. Eur. Heart J. 45, 475–484 (2024).
Patel, R. S., Gonzalez, M. D., Ajibawo, T. & Baweja, R. Cannabis use disorder and increased risk of arrhythmia-related hospitalization in young adults. Am. J. Addict. 30, 578–584 (2021).
Chouairi, F. et al. Cannabis use disorder among atrial fibrillation admissions, 2008-2018. Pacing Clin. Electrophysiol. 44, 1934–1938 (2021).
Gillett, L. et al. Arrhythmic effects of cannabis in ischemic heart disease. Cannabis Cannabinoid Res. 8, 867–876 (2023).
Richards, J. R. Mechanisms for the risk of acute coronary syndrome and arrhythmia associated with phytogenic and synthetic cannabinoid use. J. Cardiovasc. Pharmacol. Ther. 25, 508–522 (2020).
Kariyanna, P. T. et al. Marijuana induced myocarditis: a new entity of toxic myocarditis. Am. J. Med. Case Rep. 6, 169–172 (2018).
Khanji, M. Y. et al. Association between recreational cannabis use and cardiac structure and function. JACC Cardiovasc. Imaging 13, 886–888 (2020).
Bene-Alhasan, Y. et al. Daily marijuana use is associated with incident heart failure: “All of Us” research program [abstract]. Circulation 148 (Suppl. 1), 13812 (2023).
Chen, B. et al. Endothelial cannabinoid CB1 receptor deficiency reduces arterial inflammation and lipid uptake in response to atheroprone shear stress. Preprint at bioRxiv, https://doi.org/10.1101/2024.05.15.594375 (2024).
Mukhopadhyay, P. et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free. Radic. Biol. Med. 50, 179–195 (2011).
Slavic, S. et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. 91, 811–823 (2013).
Defer, N. et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 23, 2120–2130 (2009).
McPartland, J. M., Duncan, M., Di Marzo, V. & Pertwee, R. G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 172, 737–753 (2015).
Alfulaij, N. et al. Cannabinoids, the heart of the matter. J. Am. Heart Assoc. 7, e009099 (2018).
Lee, W. S. et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol. Med. 22, 136–146 (2016).
Hao, E. et al. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 21, 38–45 (2015).
Rajesh, M. et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 56, 2115–2125 (2010).
Hampson, A. J., Grimaldi, M., Axelrod, J. & Wink, D. Cannabidiol and (–)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl Acad. Sci. USA 95, 8268–8273 (1998).
Pacher, P., Kogan, N. M. & Mechoulam, R. Beyond THC and endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 60, 637–659 (2020).
Gonca, E. & Darici, F. The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: the role of adenosine A1 receptors. J. Cardiovasc. Pharmacol. Ther. 20, 76–83 (2015).
Stanley, C. P., Hind, W. H., Tufarelli, C. & O’Sullivan, S. E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 107, 568–578 (2015).
Kumric, M., Bozic, J., Dujic, G., Vrdoljak, J. & Dujic, Z. Chronic effects of effective oral cannabidiol delivery on 24-h ambulatory blood pressure and vascular outcomes in treated and untreated hypertension (HYPER-H21-4): study protocol for a randomized, placebo-controlled, and crossover study. J. Pers. Med. 12, 1037 (2022).
National Conference of State Legislatures. State Medical Cannabis Laws NCSL www.ncsl.org/health/state-medical-cannabis-laws (2024).
Balachandran, P., Elsohly, M. & Hill, K. P. Cannabidiol interactions with medications, illicit substances, and alcohol: a comprehensive review. J. Gen. Intern. Med. 36, 2074–2084 (2021).
Moore, A., Straube, S., Fisher, E. & Eccleston, C. Cannabidiol (CBD) products for pain: ineffective, expensive, and with potential harms. J. Pain 25, 833–842 (2024).
Bhat, T. A., Kalathil, S. G., Goniewicz, M. L., Hutson, A. & Thanavala, Y. Not all vaping is the same: differential pulmonary effects of vaping cannabidiol versus nicotine. Thorax 78, 922–932 (2023).
Rossheim, M. E., LoParco, C. R., Henry, D., Trangenstein, P. J. & Walters, S. T. Delta-8, delta-10, HHC, THC-O, THCP, and THCV: what should we call these products? J. Stud. Alcohol. Drugs 84, 357–360 (2023).
O’Mahony, B., O’Malley, A., Kerrigan, O. & McDonald, C. HHC-induced psychosis: a case series of psychotic illness triggered by a widely available semisynthetic cannabinoid. Ir. J. Psychol. Med. https://doi.org/10.1017/ipm.2024.3 (2024).
Harlow, A. F., Miech, R. A. & Leventhal, A. M. Adolescent Δ8-THC and marijuana use in the US. JAMA 331, 861–865 (2024).
Govindarajan, R. K. et al. Biosynthesis of phytocannabinoids and structural insights: a review. Metabolites 13, 442 (2023).
Raup-Konsavage, W. M. et al. Efficient synthesis for altering side chain length on cannabinoid molecules and their effects in chemotherapy and chemotherapeutic induced neuropathic pain. Biomolecules 12, 1869 (2022).
Nachnani, R., Raup-Konsavage, W. M. & Vrana, K. E. The pharmacological case for cannabigerol. J. Pharmacol. Exp. Ther. 376, 204–212 (2021).
Peters, E. N. et al. Pharmacokinetics of cannabichromene in a medical cannabis product also containing cannabidiol and Δ9-tetrahydrocannabinol: a pilot study. Eur. J. Clin. Pharmacol. 78, 259–265 (2022).
Abioye, A. et al. Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2, 6 (2020).
Jadoon, K. A. et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 39, 1777–1786 (2016).
Dziemitko, S., Harasim-Symbor, E. & Chabowski, A. How do phytocannabinoids affect cardiovascular health? An update on the most common cardiovascular diseases. Ther. Adv. Chronic Dis. 14, 20406223221143239 (2023).
DeFilippis, E. M. et al. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 75, 320–332 (2020).
Bedi, G., Cooper, Z. D. & Haney, M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict. Biol. 18, 872–881 (2013).
Zongo, A. et al. Medical cannabis authorization and the risk of cardiovascular events: a longitudinal cohort study. BMC Cardiovasc. Disord. 21, 426 (2021).
Devinsky, O. et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 378, 1888–1897 (2018).
Devinsky, O. et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 376, 2011–2020 (2017).
Ahmed, A. et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int. J. Cardiol. 123, 138–146 (2008).
Lee, K. et al. Cannabidiol exposure during gestation leads to adverse cardiac outcomes early in postnatal life in male rat offspring. Cannabis Cannabinoid Res. 9, 781–796 (2024).
D’hooghe, M. et al. Sativex(R) (nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: the Belgian experience. BMC Neurol. 21, 227 (2021).
Nurmikko, T. J. et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 133, 210–220 (2007).
Adams, A. J. et al. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 376, 235–242 (2017).
Fantegrossi, W. E., Moran, J. H., Radominska-Pandya, A. & Prather, P. L. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ9-THC: mechanism underlying greater toxicity? Life Sci. 97, 45–54 (2014).
Hermanns-Clausen, M. et al. Acute side effects after consumption of the new synthetic cannabinoids AB-CHMINACA and MDMB-CHMICA. Clin. Toxicol. 56, 404–411 (2018).
Mir, A., Obafemi, A., Young, A. & Kane, C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 128, e1622–e1627 (2011).
Jayakumar, N. et al. Co-use and mixing tobacco with cannabis among Ontario adults. Nicotine Tob. Res. 23, 171–178 (2021).
Wu, T. C., Tashkin, D. P., Djahed, B. & Rose, J. E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 318, 347–351 (1988).
Hancox, R. J. et al. Effects of cannabis on lung function: a population-based cohort study. Eur. Respir. J. 35, 42–47 (2010).
Tan, W. C. et al. The effects of marijuana smoking on lung function in older people. Eur. Respir. J. 54, 1900826 (2019).
Crosland, B. A. et al. Risk of adverse neonatal outcomes after combined prenatal cannabis and nicotine exposure. JAMA Netw. Open. 7, e2410151 (2024).
Hammond, C. J. et al. Co-occurring tobacco and cannabis use in adolescents: dissociable relationships with mediofrontal electrocortical activity during reward feedback processing. Neuroimage Clin. 30, 102592 (2021).
Vogel, E. A., Rubinstein, M. L., Prochaska, J. J. & Ramo, D. E. Associations between marijuana use and tobacco cessation outcomes in young adults. J. Subst. Abuse Treat. 94, 69–73 (2018).
Panlilio, L. V., Solinas, M., Matthews, S. A. & Goldberg, S. R. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology 32, 646–657 (2007).
Bierut, L. J. et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch. Gen. Psychiatry 55, 982–988 (1998).
Hartz, S. M. et al. Increased genetic vulnerability to smoking at CHRNA5 in early-onset smokers. Arch. Gen. Psychiatry 69, 854–860 (2012).
Lubke, G. H., Stephens, S. H., Lessem, J. M., Hewitt, J. K. & Ehringer, M. A. The CHRNA5/A3/B4 gene cluster and tobacco, alcohol, cannabis, inhalants and other substance use initiation: replication and new findings using mixture analyses. Behav. Genet. 42, 636–646 (2012).
Ibsen, M. S., Connor, M. & Glass, M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2, 48–60 (2017).
Cheung, C. P. et al. Cannabis inhalation acutely reduces muscle sympathetic nerve activity in humans. Circulation 146, 1972–1974 (2022).
Acknowledgements
The authors are supported by the Heart and Stroke Barnett-Ivey Chair (to M.C.), the Tobacco-Related Disease Research Program (TRDRP, T34FT8070) and the American Heart Association (AHA, 23DIVSUP1076489) (to N.J.-T.), the Stanford Cardiovascular Institute, TRDRP (27IR-0012), Gootter-Jensen Foundation and the AHA (20YVNR3500014) (to J.C.W.).
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.C. is a consultant for Greenstone Biosciences. J.C.W. is a co-founder and on the Scientific Advisory Board of Greenstone Biosciences. N.J.-T. declares no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Emilie Jouanjus, George Kunos and Pal Pacher for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandy, M., Jimenez-Tellez, N. & Wu, J.C. The relationship between cannabis and cardiovascular disease: clearing the haze. Nat Rev Cardiol 22, 467–481 (2025). https://doi.org/10.1038/s41569-025-01121-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-025-01121-6
This article is cited by
-
4F-MDMB-BICA toxicity: a fatal case and literature review of synthetic cannabinoid fatalities
Forensic Science, Medicine and Pathology (2026)