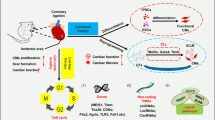
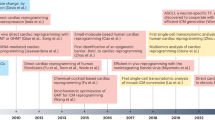
Abstract
Irreversible cardiac fibrosis, cardiomyocyte death and chronic cardiac dysfunction after myocardial infarction pose a substantial global health-care challenge, with no curative treatments available. To regenerate the injured heart, cardiomyocytes must proliferate to replace lost myocardial tissue — a capability that adult mammals have largely forfeited to adapt to the demanding conditions of life. Using various preclinical models, our understanding of cardiomyocyte proliferation has progressed remarkably, leading to the successful reactivation of cell cycle induction in adult animals, with functional recovery after cardiac injury. Central to this success is the targeting of key pathways and structures that drive cardiomyocyte maturation after birth — nucleation and ploidy, sarcomere structure, developmental signalling, chromatin and epigenetic regulation, the microenvironment and metabolic maturation — forming a complex regulatory framework that allows efficient cellular contraction but restricts cardiomyocyte proliferation. In this Review, we explore the molecular pathways underlying these core mechanisms and how their manipulation can reactivate the cell cycle in cardiomyocytes, potentially contributing to cardiac repair.
Key points
-
The capacity for mammalian cardiomyocyte proliferation is rapidly lost shortly after birth to make way for an antiproliferative maturation programme that is necessary for survival and to sustain adult life.
-
Manipulation of this maturation process holds the potential to trigger cardiomyocyte proliferation in adult animals, providing a foundation for regenerative strategies that could improve healing after injury.
-
Six major influences on cardiomyocyte maturation are nucleation and ploidy, sarcomere structure, developmental signalling, epigenetic regulation, the microenvironment and metabolic maturation; targeted manipulation can yield diverse effects on the proliferative capacity of adult cardiomyocytes.
-
Metabolic interventions that promote increased glucose utilization with a concomitant reduction in oxidative phosphorylation seem to be both safe and effective in unlocking adult cardiomyocyte proliferation.
-
Epigenetic strategies can be similarly effective, but encompass a wide array of regulatory responses, for which our understanding remains limited.
-
The cardiac microenvironment and the non-parenchymal cell population in the heart are crucial to guiding the adult cardiomyocyte proliferative response, whereby transient inflammatory and senescence signalling events shape an environment that is conducive to proliferation events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guo, Y. & Pu, W. T. Cardiomyocyte maturation: new phase in development. Circ. Res. 126, 1086–1106 (2020).
Ikenishi, A. et al. Cell cycle regulation in mouse heart during embryonic and postnatal stages. Dev. Growth Differ. 54, 731–738 (2012).
Engel, F. B., Schebesta, M. & Keating, M. T. Anillin localization defect in cardiomyocyte binucleation. J. Mol. Cell Cardiol. 41, 601–612 (2006).
Zebrowski, D. C. et al. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. eLife 4, e05563 (2015).
Vivien, C. J., Hudson, J. E. & Porrello, E. R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 1, 16012 (2016).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Alkass, K. et al. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell 163, 1026–1036 (2015).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Gilsbach, R. et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat. Commun. 5, 5288 (2014).
Gilsbach, R. et al. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 9, 391 (2018).
Lacraz, G. P. A. et al. Tomo-seq identifies SOX9 as a key regulator of cardiac fibrosis during ischemic injury. Circulation 136, 1396–1409 (2017).
Pandey, P. et al. Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev. Cell 44, 326–336.e3 (2018).
van Duijvenboden, K. et al. Conserved NPPB+ border zone switches from MEF2- to AP-1-driven gene program. Circulation 140, 864–879 (2019).
Wu, C.-C. et al. Spatially resolved genome-wide transcriptional profiling identifies BMP signaling as essential regulator of zebrafish cardiomyocyte regeneration. Dev. Cell 36, 36–49 (2016).
Zhang, Y. et al. Single-cell imaging and transcriptomic analyses of endogenous cardiomyocyte dedifferentiation and cycling. Cell Discov. 5, 30 (2019).
Bradley, L. A., Young, A., Li, H., Billcheck, H. O. & Wolf, M. J. Loss of endogenously cycling adult cardiomyocytes worsens myocardial function. Circ. Res. 128, 155–168 (2021).
Maass, K., Chase, S. E., Lin, X. & Delmar, M. Cx43 CT domain influences infarct size and susceptibility to ventricular tachyarrhythmias in acute myocardial infarction. Cardiovasc. Res. 84, 361–367 (2009).
Wang, W. E. et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 136, 834–848 (2017).
Khan, M. S. et al. Global epidemiology of heart failure. Nat. Rev. Cardiol. 21, 717–734 (2024).
Zhang, H. et al. AP-1 activation mediates post-natal cardiomyocyte maturation. Cardiovasc. Res. 119, 536–550 (2023).
Reuter, S. P. et al. Cardiac troponin I-interacting kinase affects cardiomyocyte S-phase activity but not cardiomyocyte proliferation. Circulation 147, 142–153 (2023).
Yin, K. et al. Polyploidisation pleiotropically buffers ageing in hepatocytes. J. Hepatol. 81, 289–302 (2024).
Østergaard, K. H. et al. Left ventricular morphology of the giraffe heart examined by stereological methods. Anat. Rec. 296, 611–621 (2013).
Velayutham, N. et al. Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine. J. Mol. Cell Cardiol. 146, 95–108 (2020).
Patterson, M. et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat. Genet. 49, 1346–1353 (2017).
Chen, F. et al. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 26, 27–33.e4 (2020).
Poolman, R. A. & Brooks, G. Expressions and activities of cell cycle regulatory molecules during the transition from myocyte hyperplasia to hypertrophy. J. Mol. Cell Cardiol. 30, 2121–2135 (1998).
Li, F., Wang, X., Bunger, P. C. & Gerdes, A. M. Formation of binucleated cardiac myocytes in rat heart: I. Role of actin-myosin contractile ring. J. Mol. Cell Cardiol. 29, 1541–1551 (1997).
Li, F., Wang, X. & Gerdes, A. M. Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation. J. Mol. Cell Cardiol. 29, 1553–1565 (1997).
Aix, E., Gutiérrez-Gutiérrez, Ó., Sánchez-Ferrer, C., Aguado, T. & Flores, I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J. Cell Biol. 213, 571–583 (2016).
Liu, Z., Yue, S., Chen, X., Kubin, T. & Braun, T. Regulation of cardiomyocyte polyploidy and multinucleation by cyclinG1. Circ. Res. 106, 1498–1506 (2010).
González-Rosa, J. M. et al. Myocardial polyploidization creates a barrier to heart regeneration in zebrafish. Dev. Cell 44, 433–446.e7 (2018).
Aix, E. et al. Telomeres fuse during cardiomyocyte maturation. Circulation 147, 1634–1636 (2023).
Derks, W. & Bergmann, O. Polyploidy in cardiomyocytes: roadblock to heart regeneration? Circ. Res. 126, 552–565 (2020).
Hirose, K. et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 364, 184–188 (2019).
Rigaud, V. O. C. et al. RNA-binding protein LIN28a regulates new myocyte formation in the heart through long noncoding RNA-H19. Circulation 147, 324–337 (2023).
Pettinato, A. M. et al. Sarcomere function activates a p53-dependent DNA damage response that promotes polyploidization and limits in vivo cell engraftment. Cell Rep. 35, 109088 (2021).
Windmueller, R. et al. Direct comparison of mononucleated and binucleated cardiomyocytes reveals molecular mechanisms underlying distinct proliferative competencies. Cell Rep. 30, 3105–3116.e4 (2020).
Yekelchyk, M., Guenther, S., Preussner, J. & Braun, T. Mono- and multi-nucleated ventricular cardiomyocytes constitute a transcriptionally homogenous cell population. Basic. Res. Cardiol. 114, 36 (2019).
Hesse, M. et al. Proximity to injury, but neither number of nuclei nor ploidy define pathological adaptation and plasticity in cardiomyocytes. J. Mol. Cell Cardiol. 152, 95–104 (2021).
Yu, Z. et al. Increasing mononuclear diploid cardiomyocytes by loss of E2F transcription factor 7/8 fails to improve cardiac regeneration after infarct. Circulation 147, 183–186 (2023).
Swift, S. K. et al. Cardiomyocyte ploidy is dynamic during postnatal development and varies across genetic backgrounds. Development 150, dev201318 (2023).
Akins, K. A. et al. Runx1 is sufficient but not required for cardiomyocyte cell-cycle activation. Am. J. Physiol. Heart Circ. Physiol. 327, H377–H389 (2024).
Liao, H. S. et al. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ. Res. 88, 443–450 (2001).
Karsenty, C. et al. Ephrin-B1 regulates the adult diastolic function through a late postnatal maturation of cardiomyocyte surface crests. eLife 12, e80904 (2023).
Siedner, S. et al. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J. Physiol. 548, 493–505 (2003).
Guo, Y. et al. Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor. Nat. Commun. 9, 3837 (2018).
Anversa, P., Olivetti, G. & Loud, A. V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ. Res. 46, 495–502 (1980).
Taubenberger, A. V., Baum, B. & Matthews, H. K. The mechanics of mitotic cell rounding. Front. Cell Dev. Biol. 8, 687 (2020).
Buikema, J. W. et al. Wnt activation and reduced cell-cell contact synergistically induce massive expansion of functional human iPSC-derived cardiomyocytes. Cell Stem Cell 27, 50–63.e5 (2020).
Yahalom-Ronen, Y., Rajchman, D., Sarig, R., Geiger, B. & Tzahor, E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife 4, e07455 (2015).
Ahuja, P., Perriard, E., Perriard, J.-C. & Ehler, E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J. Cell Sci. 117, 3295–3306 (2004).
Engel, F. B. et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes. Dev. 19, 1175–1187 (2005).
Abouleisa, R. R. E. et al. Transient cell cycle induction in cardiomyocytes to treat subacute ischemic heart failure. Circulation 145, 1339–1355 (2022).
Monroe, T. O. et al. YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in vivo. Dev. Cell 48, 765–779.e7 (2019).
Morikawa, Y. et al. YAP overcomes mechanical barriers to induce mitotic rounding and adult cardiomyocyte division. Circulation 151, 76–93 (2025).
Leach, J. P. et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550, 260–264 (2017).
Liu, S. et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 13, eabd6892 (2021).
D’Uva, G. et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 (2015).
Mills, R. J. et al. Drug screening in human PSC-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell 24, 895–907.e6 (2019).
El-Nachef, D. et al. Engrafted human induced pluripotent stem cell-derived cardiomyocytes undergo clonal expansion in vivo. Circulation 143, 1635–1638 (2021).
Liu, H. et al. Control of cytokinesis by β-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Sci. Transl. Med. 11, eaaw6419 (2019).
Sallin, P., de Preux Charles, A.-S., Duruz, V., Pfefferli, C. & Jaźwińska, A. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Dev. Biol. 399, 27–40 (2015).
Aballo, T. J. et al. Integrated proteomics identifies troponin i isoform switch as a regulator of a sarcomere-metabolism axis during cardiac regeneration. Preprint at bioRxiv https://doi.org/10.1101/2023.10.20.563389 (2023).
Bailey, L. R. J. et al. MBNL1 regulates programmed postnatal switching between regenerative and differentiated cardiac states. Circulation 149, 1812–1829 (2024).
Xiao, F. et al. Adducin regulates sarcomere disassembly during cardiomyocyte mitosis. Circulation 150, 791–805 (2024).
Fentzke, R. C. et al. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J. Physiol. 517, 143–157 (1999).
Fatkin, D. et al. Neonatal cardiomyopathy in mice homozygous for the Arg403Gln mutation in the alpha cardiac myosin heavy chain gene. J. Clin. Invest. 103, 147–153 (1999).
Nishii, K. et al. Targeted disruption of the cardiac troponin T gene causes sarcomere disassembly and defects in heartbeat within the early mouse embryo. Dev. Biol. 322, 65–73 (2008).
Metzger, J. M. The road to physiological maturation of stem cell-derived cardiac muscle runs through the sarcomere. J. Mol. Cell Cardiol. 170, 117–120 (2022).
Guo, Y. et al. Sarcomeres regulate murine cardiomyocyte maturation through MRTF-SRF signaling. Proc. Natl Acad. Sci. USA 118, e2008861118 (2021).
Htet, M. et al. A transcriptional enhancer regulates cardiac maturation. Nat. Cardiovasc. Res. 3, 666–684 (2024).
Chen, X. et al. QKI is a critical pre-mRNA alternative splicing regulator of cardiac myofibrillogenesis and contractile function. Nat. Commun. 12, 89 (2021).
Montañés-Agudo, P. et al. The RNA-binding protein QKI governs a muscle-specific alternative splicing program that shapes the contractile function of cardiomyocytes. Cardiovasc. Res. 119, 1161–1174 (2023).
Garbutt, T. A. et al. Epigenetic regulation of cardiomyocyte maturation by arginine methyltransferase CARM1. Circulation 149, 1501–1515 (2024).
Akerberg, A. A. et al. RBPMS2 is a myocardial-enriched splicing regulator required for cardiac function. Circ. Res. 131, 980–1000 (2022).
Mathiyalagan, P. et al. FTO-dependent N6-methyladenosine regulates cardiac function during remodeling and repair. Circulation 139, 518–532 (2019).
Lipov, A. et al. Exploring the complex spectrum of dominance and recessiveness in genetic cardiomyopathies. Nat. Cardiovasc. Res. 2, 1078–1094 (2023).
Guo, Y. et al. Ryanodine receptor 2 (RYR2) dysfunction activates the unfolded protein response and perturbs cardiomyocyte maturation. Cardiovasc. Res. 119, 221–235 (2023).
Purdy, A. L., Swift, S. K., Sucov, H. M. & Patterson, M. Tnni3k influences cardiomyocyte S-phase activity and proliferation. J. Mol. Cell Cardiol. 183, 22–26 (2023).
Nguyen, N. U. N. et al. A calcineurin-Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature 582, 271–276 (2020).
Ahmed, M. S. et al. Identification of FDA-approved drugs that induce heart regeneration in mammals. Nat. Cardiovasc. Res. 3, 372–388 (2024).
Maillet, M. et al. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J. Biol. Chem. 285, 6716–6724 (2010).
Fu, W. et al. Transient induction of actin cytoskeletal remodeling associated with dedifferentiation, proliferation, and redifferentiation stimulates cardiac regeneration. Acta Pharm. Sin. B 14, 2537–2553 (2024).
Sturzu, A. C. et al. Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation 132, 109–121 (2015).
Pikkarainen, S., Tokola, H., Kerkelä, R. & Ruskoaho, H. GATA transcription factors in the developing and adult heart. Cardiovasc. Res. 63, 196–207 (2004).
Akazawa, H. & Komuro, I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacol. Ther. 107, 252–268 (2005).
Hutson, M. R. et al. Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development 137, 3001–3011 (2010).
Tirosh-Finkel, L. et al. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development 137, 2989–3000 (2010).
Li, P. et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 138, 1795–1805 (2011).
Thomas, N. A., Koudijs, M., van Eeden, F. J. M., Joyner, A. L. & Yelon, D. Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development 135, 3789–3799 (2008).
Rowton, M. et al. Hedgehog signaling activates a mammalian heterochronic gene regulatory network controlling differentiation timing across lineages. Dev. Cell 57, 2181–2203.e9 (2022).
Ai, D. et al. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc. Natl Acad. Sci. USA 104, 9319–9324 (2007).
Ye, B. et al. APC controls asymmetric Wnt/β-catenin signaling and cardiomyocyte proliferation gradient in the heart. J. Mol. Cell Cardiol. 89, 287–296 (2015).
Miyamoto, M. et al. Cardiac progenitors instruct second heart field fate through Wnts. Proc. Natl Acad. Sci. USA 120, e2217687120 (2023).
Meilhac, S. M. & Buckingham, M. E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol. 15, 705–724 (2018).
Chen, H. et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131, 2219–2231 (2004).
Grego-Bessa, J. et al. Notch signaling is essential for ventricular chamber development. Dev. Cell 12, 415–429 (2007).
D’Amato, G. et al. Sequential Notch activation regulates ventricular chamber development. Nat. Cell Biol. 18, 7–20 (2016).
Cai, C.-L. et al. T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development 132, 2475–2487 (2005).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Sim, C. B. et al. Dynamic changes in the cardiac methylome during postnatal development. FASEB J. 29, 1329–1343 (2015).
Mills, R. J. et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl Acad. Sci. USA 114, E8372–E8381 (2017).
Sakamoto, T. et al. The nuclear receptor ERR cooperates with the cardiogenic factor GATA4 to orchestrate cardiomyocyte maturation. Nat. Commun. 13, 1991 (2022).
Iyer, L. M. et al. A context-specific cardiac β-catenin and GATA4 interaction influences TCF7L2 occupancy and remodels chromatin driving disease progression in the adult heart. Nucleic Acids Res. 46, 2850–2867 (2018).
Yu, W. et al. GATA4 regulates Fgf16 to promote heart repair after injury. Development 143, 936–949 (2016).
Liang, Q. et al. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J. Biol. Chem. 276, 30245–30253 (2001).
Qi, L. et al. Kindlin-2 suppresses transcription factor GATA4 through interaction with SUV39H1 to attenuate hypertrophy. Cell Death Dis. 10, 890 (2019).
Oka, T. et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 98, 837–845 (2006).
Gupta, V. et al. An injury-responsive Gata4 program shapes the zebrafish cardiac ventricle. Curr. Biol. 23, 1221–1227 (2013).
Mohammadi, M M. et al. Induction of cardiomyocyte proliferation and angiogenesis protects neonatal mice from pressure overload-associated maladaptation. JCI Insight 5, 128336 (2019).
Xiang, F.-L., Guo, M. & Yutzey, K. E. Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation 133, 1081–1092 (2016).
Chakraborty, S., Sengupta, A. & Yutzey, K. E. Tbx20 promotes cardiomyocyte proliferation and persistence of fetal characteristics in adult mouse hearts. J. Mol. Cell Cardiol. 62, 203–213 (2013).
Bongiovanni, C. et al. BMP7 promotes cardiomyocyte regeneration in zebrafish and adult mice. Cell Rep. 43, 114162 (2024).
Ebelt, H. et al. Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock 39, 353–360 (2013).
Vukicevic, S. et al. Bone morphogenetic protein 1.3 inhibition decreases scar formation and supports cardiomyocyte survival after myocardial infarction. Nat. Commun. 13, 81 (2022).
Engel, F. B., Hsieh, P. C. H., Lee, R. T. & Keating, M. T. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl Acad. Sci. USA 103, 15546–15551 (2006).
Hubert, F. et al. FGF10 promotes cardiac repair through a dual cellular mechanism increasing cardiomyocyte renewal and inhibiting fibrosis. Cardiovasc. Res. 118, 2625–2637 (2022).
Wang, Z. et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc. Natl Acad. Sci. USA 116, 18455–18465 (2019).
Bersell, K., Arab, S., Haring, B. & Kühn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009).
Reuter, S., Soonpaa, M. H., Firulli, A. B., Chang, A. N. & Field, L. J. Recombinant neuregulin 1 does not activate cardiomyocyte DNA synthesis in normal or infarcted adult mice. PLoS ONE 9, e115871 (2014).
Polizzotti, B. D. et al. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci. Transl. Med. 7, 281ra45 (2015).
De Keulenaer, G. W. et al. Mechanisms of the multitasking endothelial protein NRG-1 as a compensatory factor during chronic heart failure. Circ. Heart Fail. 12, e006288 (2019).
Zurek, M. et al. Neuregulin-1 induces cardiac hypertrophy and impairs cardiac performance in post-myocardial infarction rats. Circulation 142, 1308–1311 (2020).
Maciver, D. H. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur. J. Echocardiogr. 12, 228–234 (2011).
Aharonov, A. et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356 (2020).
DeBosch, B. et al. Akt1 is required for physiological cardiac growth. Circulation 113, 2097–2104 (2006).
Mutlak, M. et al. Extracellular signal-regulated kinase (ERK) activation preserves cardiac function in pressure overload induced hypertrophy. Int. J. Cardiol. 270, 204–213 (2018).
DeBosch, B., Sambandam, N., Weinheimer, C., Courtois, M. & Muslin, A. J. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J. Biol. Chem. 281, 32841–32851 (2006).
Kehat, I. et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ. Res. 108, 176–183 (2011).
Fujio, Y., Nguyen, T., Wencker, D., Kitsis, R. N. & Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101, 660–667 (2000).
Quaife-Ryan, G. A. et al. β-Catenin drives distinct transcriptional networks in proliferative and nonproliferative cardiomyocytes. Development 147, dev193417 (2020).
Wo, D. et al. Opposing roles of Wnt inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential targeting of LRP5/6 and β-catenin. Circulation 134, 1991–2007 (2016).
Antos, C. L. et al. Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc. Natl Acad. Sci. USA 99, 907–912 (2002).
Chen, X. et al. The β-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol. Cell Biol. 26, 4462–4473 (2006).
Hou, N. et al. Transcription factor 7-like 2 mediates canonical Wnt/β-catenin signaling and c-myc upregulation in heart failure. Circ. Heart Fail. 9, e003010 (2016).
He, A. et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 5, 4907 (2014).
Mia, M. M. et al. YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLoS Biol. 18, e3000941 (2020).
Magadum, A. et al. Live cell screening platform identifies PPARδ as a regulator of cardiomyocyte proliferation and cardiac repair. Cell Res. 27, 1002–1019 (2017).
Magadum, A. et al. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation 141, 1249–1265 (2020).
Oikonomopoulos, A. et al. Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ. Res. 109, 1363–1374 (2011).
Wu, Y. et al. LRP6 downregulation promotes cardiomyocyte proliferation and heart regeneration. Cell Res. 31, 450–462 (2021).
Singh, B. N. et al. A conserved HH-Gli1-Mycn network regulates heart regeneration from newt to human. Nat. Commun. 9, 4237 (2018).
Kawagishi, H. et al. Sonic hedgehog signaling regulates the mammalian cardiac regenerative response. J. Mol. Cell Cardiol. 123, 180–184 (2018).
Xiao, Q. et al. Impaired sonic hedgehog pathway contributes to cardiac dysfunction in type 1 diabetic mice with myocardial infarction. Cardiovasc. Res. 95, 507–516 (2012).
Kusano, K. F. et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat. Med. 11, 1197–1204 (2005).
Xin, M. et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 4, ra70 (2011).
von Gise, A. et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl Acad. Sci. USA 109, 2394–2399 (2012).
Xin, M. et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. USA 110, 13839–13844 (2013).
Heallen, T. et al. Hippo signaling impedes adult heart regeneration. Development 140, 4683–4690 (2013).
Wang, J., Liu, S., Heallen, T. & Martin, J. F. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 15, 672–684 (2018).
Liu, X. et al. Cell proliferation fate mapping reveals regional cardiomyocyte cell-cycle activity in subendocardial muscle of left ventricle. Nat. Commun. 12, 5784 (2021).
Liu, S. et al. Yap promotes noncanonical Wnt signals from cardiomyocytes for heart regeneration. Circ. Res. 129, 782–797 (2021).
Zhao, M. et al. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation 144, 210–228 (2021).
Gong, R. et al. Cyclin L1 controls cardiomyocyte proliferation and heart repair after injury. Signal. Transduct. Target. Ther. 8, 243 (2023).
Li, Y. et al. gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation 142, 967–982 (2020).
Yang, H. et al. Omentin-1 drives cardiomyocyte cell cycle arrest and metabolic maturation by interacting with BMP7. Cell Mol. Life Sci. 80, 186 (2023).
Tian, Y. et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 7, 279ra38 (2015).
Torrini, C. et al. Common regulatory pathways mediate activity of microRNAs inducing cardiomyocyte proliferation. Cell Rep. 27, 2759–2771.e5 (2019).
Nugroho, A. B. et al. Micro RNA-411 expression improves cardiac phenotype following myocardial infarction in mice. JACC Basic. Transl. Sci. 7, 859–875 (2022).
Cai, B. et al. Targeting LncDACH1 promotes cardiac repair and regeneration after myocardium infarction. Cell Death Differ. 27, 2158–2175 (2020).
Vite, A., Zhang, C., Yi, R., Emms, S. & Radice, G. L. α-Catenin-dependent cytoskeletal tension controls Yap activity in the heart. Development 145, dev149823 (2018).
Li, X., McLain, C., Samuel, M. S., Olson, M. F. & Radice, G. L. Actomyosin-mediated cellular tension promotes Yap nuclear translocation and myocardial proliferation through α5 integrin signaling. Development 150, dev201013 (2023).
Li, J. et al. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ. Res. 116, 70–79 (2015).
Guo, H. et al. Intercalated disc protein Xinβ is required for Hippo-YAP signaling in the heart. Nat. Commun. 11, 4666 (2020).
Bassat, E. et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017).
Baehr, A. et al. Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs. Circulation 142, 868–881 (2020).
Zlatanova, I. et al. An injury-responsive mmp14b enhancer is required for heart regeneration. Sci. Adv. 9, eadh5313 (2023).
Galli, G. G. et al. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol. Cell 60, 328–337 (2015).
Lin, Z. et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ. Res. 116, 35–45 (2015).
Shao, D. et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat. Commun. 5, 3315 (2014).
Tao, G. et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature 534, 119–123 (2016).
Gabisonia, K. et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422 (2019).
Morikawa, Y., Heallen, T., Leach, J., Xiao, Y. & Martin, J. F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 547, 227–231 (2017).
Liu, S. et al. Microtubules sequester acetylated YAP in the cytoplasm and inhibit heart regeneration. Circulation 151, 59–75 (2025).
Lin, Z. et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 115, 354–363 (2014).
Xiao, Y. et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes. Dev. 33, 1491–1505 (2019).
Murphy, S. A. et al. PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2. Nat. Commun. 12, 1648 (2021).
Yue, P. et al. Yap1 modulates cardiomyocyte hypertrophy via impaired mitochondrial biogenesis in response to chronic mechanical stress overload. Theranostics 12, 7009–7031 (2022).
Matsui, Y. et al. Lats2 is a negative regulator of myocyte size in the heart. Circ. Res. 103, 1309–1318 (2008).
Del Re, D. P. et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J. Biol. Chem. 288, 3977–3988 (2013).
Ikeda, S. et al. Hippo deficiency leads to cardiac dysfunction accompanied by cardiomyocyte dedifferentiation during pressure overload. Circ. Res. 124, 292–305 (2019).
Kleele, T. et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439 (2021).
Byun, J. et al. Yes-associated protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload. J. Biol. Chem. 294, 3603–3617 (2019).
Perez-Gonzalez, N. A. et al. YAP and TAZ regulate cell volume. J. Cell Biol. 218, 3472–3488 (2019).
Schmidt, M. et al. The interaction effect of cardiac and non-cardiac comorbidity on myocardial infarction mortality: a nationwide cohort study. Int. J. Cardiol. 308, 1–8 (2020).
Weston, W. A. & Barr, A. R. A cell cycle centric view of tumour dormancy. Br. J. Cancer 129, 1535–1545 (2023).
Kwon, J. S. et al. Controlling depth of cellular quiescence by an Rb-E2F network switch. Cell Rep. 20, 3223–3235 (2017).
Felician, G. et al. Epigenetic modification at Notch responsive promoters blunts efficacy of inducing notch pathway reactivation after myocardial infarction. Circ. Res. 115, 636–649 (2014).
Zhou, P. et al. Dynamic changes in P300 enhancers and enhancer-promoter contacts control mouse cardiomyocyte maturation. Dev. Cell 58, 898–914.e7 (2023).
Stergachis, A. B. et al. Developmental fate and cellular maturity encoded in human regulatory DNA landscapes. Cell 154, 888–903 (2013).
Sim, C. B. et al. Sex-specific control of human heart maturation by the progesterone receptor. Circulation 143, 1614–1628 (2021).
Li, Z. et al. Postnatal state transition of cardiomyocyte as a primary step in heart maturation. Protein Cell 13, 842–862 (2022).
Cui, M. et al. Dynamic transcriptional responses to injury of regenerative and non-regenerative cardiomyocytes revealed by single-nucleus RNA sequencing. Dev. Cell 53, 102–116.e8 (2020).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Nothjunge, S. et al. DNA methylation signatures follow preformed chromatin compartments in cardiac myocytes. Nat. Commun. 8, 1667 (2017).
Greco, C. M. et al. DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy. Nat. Commun. 7, 12418 (2016).
Trivedi, C. M., Lu, M. M., Wang, Q. & Epstein, J. A. Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J. Biol. Chem. 283, 26484–26489 (2008).
Han, Z. et al. ALKBH5 regulates cardiomyocyte proliferation and heart regeneration by demethylating the mRNA of YTHDF1. Theranostics 11, 3000–3016 (2021).
Liu, X.-H. et al. Co-effects of m6A and chromatin accessibility dynamics in the regulation of cardiomyocyte differentiation. Epigenetics Chromatin 16, 32 (2023).
Deng, S. et al. RNA m6A regulates transcription via DNA demethylation and chromatin accessibility. Nat. Genet. 54, 1427–1437 (2022).
Quaife-Ryan, G. A., Sim, C. B., Porrello, E. R. & Hudson, J. E. Resetting the epigenome for heart regeneration. Semin. Cell Dev. Biol. 58, 2–13 (2016).
Ponnusamy, M. et al. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation 139, 2668–2684 (2019).
Liu, X.-M., Mao, Y., Wang, S., Zhou, J. & Qian, S.-B. METTL3 modulates chromatin and transcription dynamics during cell fate transition. Cell Mol. Life Sci. 79, 559 (2022).
Jiang, F.-Q. et al. Mettl3-mediated m6A modification of Fgf16 restricts cardiomyocyte proliferation during heart regeneration. eLife 11, e77014 (2022).
Dorn, L. E. et al. The N6-methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation 139, 533–545 (2019).
Cho, K.-W. et al. Polycomb group protein CBX7 represses cardiomyocyte proliferation through modulation of the TARDBP/RBM38 axis. Circulation 147, 1823–1842 (2023).
Boogerd, C. J. et al. Cardiomyocyte proliferation is suppressed by ARID1A-mediated YAP inhibition during cardiac maturation. Nat. Commun. 14, 4716 (2023).
Bouwman, M. et al. Cross-species comparison reveals that Hmga1 reduces H3K27me3 levels to promote cardiomyocyte proliferation and cardiac regeneration. Nat. Cardiovasc. Res. 4, 64–82 (2025).
Shi, Y. et al. α-Ketoglutarate promotes cardiomyocyte proliferation and heart regeneration after myocardial infarction. Nat. Cardiovasc. Res. 3, 1083–1097 (2024).
Li, X. et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 622, 619–626 (2023).
Ji, X. et al. Sphingolipid metabolism controls mammalian heart regeneration. Cell Metab. 36, 839–856.e8 (2024).
Bywater, M. J. et al. Reactivation of Myc transcription in the mouse heart unlocks its proliferative capacity. Nat. Commun. 11, 1827 (2020).
Sano, M. et al. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat. Med. 8, 1310–1317 (2002).
Boikova, A. et al. A transient modified mRNA encoding Myc and Cyclin T1 induces cardiac regeneration and improves cardiac function after myocardial injury. Preprint at bioRxiv https://doi.org/10.1101/2023.08.02.551469 (2023).
Gao, R. et al. Pioneering function of Isl1 in the epigenetic control of cardiomyocyte cell fate. Cell Res. 29, 486–501 (2019).
Stone, N. R. et al. Context-specific transcription factor functions regulate epigenomic and transcriptional dynamics during cardiac reprogramming. Cell Stem Cell 25, 87–102.e9 (2019).
Mellis, I. A. et al. Responsiveness to perturbations is a hallmark of transcription factors that maintain cell identity in vitro. Cell Syst. 12, 885–899.e8 (2021).
DeLaughter, D. M. et al. Single-cell resolution of temporal gene expression during heart development. Dev. Cell 39, 480–490 (2016).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Ivey, M. J. et al. Resident fibroblast expansion during cardiac growth and remodeling. J. Mol. Cell Cardiol. 114, 161–174 (2018).
Wang, Z. et al. Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. Cell Rep. 33, 108472 (2020).
Kuppe, C. et al. Spatial multi-omic map of human myocardial infarction. Nature 608, 766–777 (2022).
Liu, X. et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 588, 705–711 (2020).
Bai, Y. et al. EphrinB2-mediated CDK5/ISL1 pathway enhances cardiac lymphangiogenesis and alleviates ischemic injury by resolving post-MI inflammation. Signal. Transduct. Target. Ther. 9, 326 (2024).
Mahmoud, A. I. et al. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev. Cell 34, 387–399 (2015).
Tampakakis, E. et al. Heart neurons use clock genes to control myocyte proliferation. Sci. Adv. 7, eabh4181 (2021).
Hortells, L. et al. A specialized population of periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation. Proc. Natl Acad. Sci. USA 117, 21469–21479 (2020).
Kaur, H. et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res. 118, 1906–1917 (2016).
Kuwabara, J. T. et al. Regulation of extracellular matrix composition by fibroblasts during perinatal cardiac maturation. J. Mol. Cell Cardiol. 169, 84–95 (2022).
Wang, Y. et al. Single-cell analysis of murine fibroblasts identifies neonatal to adult switching that regulates cardiomyocyte maturation. Nat. Commun. 11, 2585 (2020).
Notari, M. et al. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 4, eaao5553 (2018).
Williams, C., Quinn, K. P., Georgakoudi, I. & Black, L. D. 3rd Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 10, 194–204 (2014).
Koopmans, T. et al. Ischemic tolerance and cardiac repair in the spiny mouse (Acomys). NPJ Regen. Med. 6, 78 (2021).
Garcia-Puig, A. et al. Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration. Mol. Cell Proteom. 18, 1745–1755 (2019).
Jacot, J. G., Martin, J. C. & Hunt, D. L. Mechanobiology of cardiomyocyte development. J. Biomech. 43, 93–98 (2010).
Yokota, T. et al. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell 182, 545–562.e23 (2020).
Li, S. et al. Cardiomyocytes disrupt pyrimidine biosynthesis in nonmyocytes to regulate heart repair. J. Clin. Invest. 132, e149711 (2022).
Valiente-Alandi, I. et al. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation 138, 1236–1252 (2018).
Wu, C.-C. et al. Modulation of mammalian cardiomyocyte cytokinesis by the extracellular matrix. Circ. Res. 127, 896–907 (2020).
Feng, J. et al. Versican promotes cardiomyocyte proliferation and cardiac repair. Circulation 149, 1004–1015 (2024).
Ieda, M. et al. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev. Cell 16, 233–244 (2009).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014).
Bajpai, G. et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ. Res. 124, 263–278 (2019).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Paddock, S. J. et al. IL4Rα signaling promotes neonatal cardiac regeneration and cardiomyocyte cell cycle activity. J. Mol. Cell Cardiol. 161, 62–74 (2021).
Zlatanova, I. et al. Iron regulator hepcidin impairs macrophage-dependent cardiac repair after injury. Circulation 139, 1530–1547 (2019).
Li, J. et al. Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner. Theranostics 9, 4324–4341 (2019).
Tan, Y., Duan, X., Wang, B., Liu, X. & Zhan, Z. Murine neonatal cardiac B cells promote cardiomyocyte proliferation and heart regeneration. NPJ Regen. Med. 8, 7 (2023).
Dolejsi, T. et al. Adult T-cells impair neonatal cardiac regeneration. Eur. Heart J. 43, 2698–2709 (2022).
Wang, G. et al. “Default” generation of neonatal regulatory T cells. J. Immunol. 185, 71–78 (2010).
Vargas Aguilar, S. et al. The PD-1–PD-L1 pathway maintains an immunosuppressive environment essential for neonatal heart regeneration. Nat. Cardiovasc. Res. 3, 389–402 (2024).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Zacchigna, S. et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat. Commun. 9, 2432 (2018).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Sarig, R. et al. Transient p53-mediated regenerative senescence in the injured heart. Circulation 139, 2491–2494 (2019).
Zhang, L. et al. Egr1 regulates regenerative senescence and cardiac repair. Nat. Cardiovasc. Res. 3, 915–932 (2024).
Feng, T. et al. CCN1-induced cellular senescence promotes heart regeneration. Circulation 139, 2495–2498 (2019).
Vagnozzi, R. J. et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409 (2020).
Han, C. et al. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 25, 1137–1151 (2015).
Velayutham, N. et al. P53 activation promotes maturational characteristics of pluripotent stem cell-derived cardiomyocytes in 3-dimensional suspension culture via FOXO-FOXM1 regulation. J. Am. Heart Assoc. 13, e033155 (2024).
Chen, X. et al. p53-dependent mitochondrial compensation in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 11, e024582 (2022).
Mosteiro, L. et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445 (2016).
Kubin, T. et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9, 420–432 (2011).
Abe, H. et al. Macrophage hypoxia signaling regulates cardiac fibrosis via Oncostatin M. Nat. Commun. 10, 2824 (2019).
Müller, J. et al. Interleukin-6-dependent phenotypic modulation of cardiac fibroblasts after acute myocardial infarction. Basic. Res. Cardiol. 109, 440 (2014).
Shin, K. et al. Harnessing the regenerative potential of interleukin11 to enhance heart repair. Nat. Commun. 15, 9666 (2024).
Broch, K. et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855 (2021).
Li, R. G. et al. YAP induces a neonatal-like pro-renewal niche in the adult heart. Nat. Cardiovasc. Res. 3, 283–300 (2024).
Kim, E. E. et al. The transcription factor EBF1 non-cell-autonomously regulates cardiac growth and differentiation. Development 150, dev202054 (2023).
Chen, Y. et al. LDHA-mediated metabolic reprogramming promoted cardiomyocyte proliferation by alleviating ROS and inducing M2 macrophage polarization. Redox Biol. 56, 102446 (2022).
Marin-Garcia, J., Ananthakrishnan, R. & Goldenthal, M. J. Heart mitochondrial DNA and enzyme changes during early human development. Mol. Cell Biochem. 210, 47–52 (2000).
Warshaw, J. B. & Terry, M. L. Cellular energy metabolism during fetal development. II. Fatty acid oxidation by the developing heart. J. Cell Biol. 44, 354–360 (1970).
Lopaschuk, G. D., Collins-Nakai, R. L. & Itoi, T. Developmental changes in energy substrate use by the heart. Cardiovasc. Res. 26, 1172–1180 (1992).
Menendez-Montes, I. et al. Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Dev. Cell 39, 724–739 (2016).
Neary, M. T. et al. Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J. Mol. Cell Cardiol. 74, 340–352 (2014).
Vega, R. B., Horton, J. L. & Kelly, D. P. Maintaining ancient organelles: mitochondrial biogenesis and maturation. Circ. Res. 116, 1820–1834 (2015).
Nichtová, Z. et al. Enhanced mitochondria-SR tethering triggers adaptive cardiac muscle remodeling. Circ. Res. 132, e171–e187 (2023).
Paredes, A. et al. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature 618, 365–373 (2023).
Schraps, N. et al. Cardiomyocyte maturation alters molecular stress response capacities and determines cell survival upon mitochondrial dysfunction. Free. Radic. Biol. Med. 213, 248–265 (2024).
Puente, B. N. et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963).
Nakada, Y. et al. Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227 (2017).
Jopling, C., Suñé, G., Faucherre, A., Fabregat, C. & Izpisua Belmonte, J. C. Hypoxia induces myocardial regeneration in zebrafish. Circulation 126, 3017–3027 (2012).
Johnson, J. et al. Systemic hypoxemia induces cardiomyocyte hypertrophy and right ventricular specific induction of proliferation. Circ. Res. 132, 723–740 (2023).
Ye, L. et al. Role of blood oxygen saturation during post-natal human cardiomyocyte cell cycle activities. JACC Basic. Transl. Sci. 5, 447–460 (2020).
Rigaud, V. O. et al. UCP2 modulates cardiomyocyte cell cycle activity, acetyl-CoA, and histone acetylation in response to moderate hypoxia. JCI Insight 7, e155475 (2022).
Kimura, W. et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 523, 226–230 (2015).
Zou, J. et al. Neddylation is required for perinatal cardiac development through stimulation of metabolic maturation. Cell Rep. 42, 112018 (2023).
Fan, Q. et al. Depletion of endothelial prolyl hydroxylase domain protein 2 and 3 promotes cardiomyocyte proliferation and prevents ventricular failure induced by myocardial infarction. Circulation 140, 440–442 (2019).
Kaelin, W. G. J. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Blasco, N. et al. Involvement of the mitochondrial nuclease EndoG in the regulation of cell proliferation through the control of reactive oxygen species. Redox Biol. 37, 101736 (2020).
McDermott-Roe, C. et al. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 478, 114–118 (2011).
Cohen, E. D. et al. Neonatal hyperoxia inhibits proliferation and survival of atrial cardiomyocytes by suppressing fatty acid synthesis. JCI Insight 6, e140785 (2021).
Cui, M. et al. Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance. Nat. Commun. 12, 5270 (2021).
Zhang, D. et al. Mitochondrial cardiomyopathy caused by elevated reactive oxygen species and impaired cardiomyocyte proliferation. Circ. Res. 122, 74–87 (2018).
Waypa, G. B. et al. Mitochondria regulate proliferation in adult cardiac myocytes. J. Clin. Invest. 134, e165482 (2024).
Cardoso, A. C. et al. Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat. Metab. 2, 167–178 (2020).
Gao, F. et al. Reduced mitochondrial protein translation promotes cardiomyocyte proliferation and heart regeneration. Circulation 148, 1887–1906 (2023).
Pianca, N. et al. Glucocorticoid receptor antagonization propels endogenous cardiomyocyte proliferation and cardiac regeneration. Nat. Cardiovasc. Res. 1, 617–633 (2022).
Bae, J. et al. Malonate promotes adult cardiomyocyte proliferation and heart regeneration. Circulation 143, 1973–1986 (2021).
Luo, X. et al. The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 12, 328 (2021).
Chong, D. et al. Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming. Cell Discov. 8, 106 (2022).
Cheng, Y.-Y. et al. Metabolic changes associated with cardiomyocyte dedifferentiation enable adult mammalian cardiac regeneration. Circulation 146, 1950–1967 (2022).
Abouleisa, R. R. E. et al. Cell cycle induction in human cardiomyocytes is dependent on biosynthetic pathway activation. Redox Biol. 46, 102094 (2021).
Mohamed, T. M. A. et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e12 (2018).
Zhu, Y. et al. Asparagine synthetase marks a distinct dependency threshold for cardiomyocyte dedifferentiation. Circulation 149, 1833–1851 (2024).
Chen, Y. et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 373, 1537–1540 (2021).
Williams, A. L. et al. HIF1 mediates a switch in pyruvate kinase isoforms after myocardial infarction. Physiol. Genomics 50, 479–494 (2018).
Hauck, L., Dadson, K., Chauhan, S., Grothe, D. & Billia, F. Inhibiting the Pkm2/b-catenin axis drives in vivo replication of adult cardiomyocytes following experimental MI. Cell Death Differ. 28, 1398–1417 (2021).
Israelsen, W. J. et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155, 397–409 (2013).
Yuko, A. E. et al. LIN28a induced metabolic and redox regulation promotes cardiac cell survival in the heart after ischemic injury. Redox Biol. 47, 102162 (2021).
Li, Z. et al. The de novo purine synthesis enzyme Adssl1 promotes cardiomyocyte proliferation and cardiac regeneration. Sci. Signal. 17, eadn3285 (2024).
Nishiyama, C. et al. Prolonged myocardial regenerative capacity in neonatal opossum. Circulation 146, 125–139 (2022).
Tamai, T. et al. Rheb (Ras homologue enriched in brain)-dependent mammalian target of rapamycin complex 1 (mTORC1) activation becomes indispensable for cardiac hypertrophic growth after early postnatal period. J. Biol. Chem. 288, 10176–10187 (2013).
Garbern, J. C. et al. Inhibition of mTOR signaling enhances maturation of cardiomyocytes derived from human-induced pluripotent stem cells via p53-induced quiescence. Circulation 141, 285–300 (2020).
Paltzer, W. G. et al. mTORC1 regulates the metabolic switch of postnatal cardiomyocytes during regeneration. J. Mol. Cell Cardiol. 187, 15–25 (2024).
Du, J. et al. A small-molecule cocktail promotes mammalian cardiomyocyte proliferation and heart regeneration. Cell Stem Cell 29, 545–558.e13 (2022).
Fan, Y. et al. Phosphoproteomic analysis of neonatal regenerative myocardium revealed important roles of checkpoint kinase 1 via activating mammalian target of rapamycin c1/ribosomal protein s6 kinase b-1 pathway. Circulation 141, 1554–1569 (2020).
Shapiro, S. D. et al. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci. Transl. Med. 6, 224ra27 (2014).
Sun, J. et al. CCND2 modified mRNA activates cell cycle of cardiomyocytes in hearts with myocardial infarction in mice and pigs. Circ. Res. 133, 484–504 (2023).
Yan, R. et al. An enhancer-based gene-therapy strategy for spatiotemporal control of cargoes during tissue repair. Cell Stem Cell 30, 96–111.e6 (2023).
Riscal, R., Riquier-Morcant, B., Gadea, G. & Linares, L. K. Give and take: the reciprocal control of metabolism and cell cycle. Methods Mol. Biol. 2740, 155–168 (2024).
Shakked, A. et al. Redifferentiated cardiomyocytes retain residual dedifferentiation signatures and are protected against ischemic injury. Nat. Cardiovasc. Res. 2, 383–398 (2023).
Nguyen, P. D. et al. Interplay between calcium and sarcomeres directs cardiomyocyte maturation during regeneration. Science 380, 758–764 (2023).
Borden, A. et al. Transient introduction of miR-294 in the heart promotes cardiomyocyte cell cycle reentry after injury. Circ. Res. 125, 14–25 (2019).
Chen, J. et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 112, 1557–1566 (2013).
Huang, S. et al. Loss of super-enhancer-regulated circRNA Nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation 139, 2857–2876 (2019).
Zhu, Y. et al. Overexpression of circRNA SNRK targets miR-103-3p to reduce apoptosis and promote cardiac repair through GSK3β/β-catenin pathway in rats with myocardial infarction. Cell Death Discov. 7, 84 (2021).
Raso, A. et al. A microRNA program regulates the balance between cardiomyocyte hyperplasia and hypertrophy and stimulates cardiac regeneration. Nat. Commun. 12, 4808 (2021).
Chen, X.-Z. et al. TMEM11 regulates cardiomyocyte proliferation and cardiac repair via METTL1-mediated m7G methylation of ATF5 mRNA. Cell Death Differ. 30, 1786–1798 (2023).
Liang, T. et al. Loss of phosphatase and tensin homolog promotes cardiomyocyte proliferation and cardiac repair after myocardial infarction. Circulation 142, 2196–2199 (2020).
Valussi, M. et al. Repression of Osmr and Fgfr1 by miR-1/133a prevents cardiomyocyte dedifferentiation and cell cycle entry in the adult heart. Sci. Adv. 7, eabi6648 (2021).
Eulalio, A. et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012).
Ma, W. et al. The highly conserved PIWI-interacting RNA CRAPIR antagonizes PA2G4-mediated NF110-NF45 disassembly to promote heart regeneration in mice. Nat. Cardiovasc. Res. 4, 102–118 (2025).
Pilz, P. M. et al. Large and small animal models of heart failure with reduced ejection fraction. Circ. Res. 130, 1888–1905 (2022).
Karpurapu, A. et al. Deep learning resolves myovascular dynamics in the failing human heart. JACC Basic. Transl. Sci. 9, 674–686 (2024).
Swift, S. K. et al. A broadly applicable method for quantifying cardiomyocyte cell division identifies proliferative events following myocardial infarction. Cell Rep. Methods 4, 100860 (2024).
Zong, H., Espinosa, J. S., Su, H. H., Muzumdar, M. D. & Luo, L. Mosaic analysis with double markers in mice. Cell 121, 479–492 (2005).
Acknowledgements
E.v.R. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme (grant agreement 874764).
Author information
Authors and Affiliations
Contributions
T.K. researched data for the article, and both authors discussed its content. T.K. wrote the manuscript, and both authors reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Mauro Giacca and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koopmans, T., van Rooij, E. Molecular gatekeepers of endogenous adult mammalian cardiomyocyte proliferation. Nat Rev Cardiol 22, 857–882 (2025). https://doi.org/10.1038/s41569-025-01145-y
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41569-025-01145-y