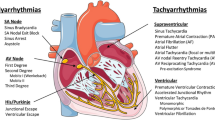
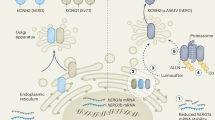
Abstract
Cardiovascular diseases are the leading cause of death globally, with cardiac arrhythmias contributing substantially to this burden. Gene therapy, which directly targets the underlying disease pathobiology, offers an appealing treatment strategy for cardiac arrhythmias owing to its potential as a one-time, curative solution. Over the past two decades, substantial efforts have been made to develop new gene therapy approaches that overcome the limitations of conventional treatments. In this Review, we describe the rationale for gene therapy to treat cardiac arrhythmias; discuss advantages and disadvantages of gene silencing, gene replacement, gene suppression-and-replacement and gene editing technologies; summarize vector modalities and delivery approaches used in the field; present examples of gene therapy strategies used for atrial and ventricular arrhythmias; and highlight the current challenges and limitations in the gene therapy field.
Key points
-
Available treatments for cardiac arrhythmias mainly focus on symptom management, fail to address the underlying disease substrate and are associated with substantial adverse effects, which makes gene therapy a promising alternative.
-
Key gene therapy strategies in cardiology involve gene silencing, gene replacement, direct genome editing and hybrid gene suppression and replacement to address various cardiac conditions at the genetic level.
-
Vector delivery poses substantial challenges in achieving targeted gene transfer to the heart while minimizing off-target effects; however, promising strategies include receptor-mediated targeting and tissue-specific promoters to improve cardiac specificity.
-
Suppression-and-replacement gene therapy is a variant-independent approach that can be effective for any monogenic dominant-negative disease, and proof-of-principle therapeutic efficacy has been demonstrated in a transgenic rabbit model of type 1 long QT syndrome.
-
Challenges such as immune responses, delivery efficiency and the limited size capacity of viral vectors remain important barriers to the successful clinical translation of gene therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on Clinical Practice Guidelines and the Heart rhythm society. Circulation 138, e210–e271 (2018).
Parameswaran, R., Al-Kaisey, A. M. & Kalman, J. M. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat. Rev. Cardiol. 18, 210–225 (2021).
Ang, R. & Earley, M. J. The role of catheter ablation in the management of atrial fibrillation. Clin. Med. 16, 267–271 (2016).
Benali, K. et al. Procedure-related complications of catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 81, 2089–2099 (2023).
Wellens, H. J. Forty years of invasive clinical electrophysiology: 1967–2007. Circ. Arrhythm. Electrophysiol. 1, 49–53 (2008).
Cingolani, E., Goldhaber, J. I. & Marban, E. Next-generation pacemakers: from small devices to biological pacemakers. Nat. Rev. Cardiol. 15, 139–150 (2018).
Infeld, M. et al. Physiological pacing for the prevention and treatment of heart failure: a state-of-the-art review. J. Card. Fail. 30, 1614–1628 (2024).
Neves, R. et al. Precision therapy in congenital long QT syndrome. Trends Cardiovasc. Med. 34, 39–47 (2024).
Sebastian, S. A. et al. Precision medicine and cardiac channelopathies: human iPSCs take the lead. Curr. Probl. Cardiol. 48, 101990 (2023).
Krahn, A. D. et al. Congenital long QT syndrome. JACC Clin. Electrophysiol. 8, 687–706 (2022).
Bergeman, A. T., Wilde, A. A. M. & van der Werf, C. Catecholaminergic polymorphic ventricular tachycardia: a review of therapeutic strategies. Card. Electrophysiol. Clin. 15, 293–305 (2023).
Schwartz, P. J. & Ackerman, M. J. Cardiac sympathetic denervation in the prevention of genetically mediated life-threatening ventricular arrhythmias. Eur. Heart J. 43, 2096–2102 (2022).
Kotta, M. C. et al. Calmodulinopathy: a novel, life-threatening clinical entity affecting the young. Front. Cardiovasc. Med. 5, 175 (2018).
Pandit, M., Finn, C., Tahir, U. A. & Frishman, W. H. Congenital long QT syndrome: a review of genetic and pathophysiologic etiologies, phenotypic subtypes, and clinical management. Cardiol. Rev. 31, 318–324 (2023).
Huang, H. et al. Mechanisms of KCNQ1 channel dysfunction in long QT syndrome involving voltage sensor domain mutations. Sci. Adv. 4, eaar2631 (2018).
Anderson, C. L. et al. Large-scale mutational analysis of Kv11.1 reveals molecular insights into type 2 long QT syndrome. Nat. Commun. 5, 5535–5535 (2014).
Mehta, A. et al. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur. Heart J. 39, 1446–1455 (2017).
Helms, A. S., Thompson, A. D. & Day, S. M. Translation of new and emerging therapies for genetic cardiomyopathies. JACC Basic. Transl. Sci. 7, 70–83 (2022).
Rinaldi, C. & Wood, M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 14, 9–21 (2018).
Di Fusco, D. et al. Antisense oligonucleotide: basic concepts and therapeutic application in inflammatory bowel disease. Front. Pharmacol. 10, 305 (2019).
Migliorati, J. M. et al. Absorption, distribution, metabolism, and excretion of US Food and Drug Administration-approved antisense oligonucleotide drugs. Drug. Metab. Dispos. 50, 888–897 (2022).
Cirak, S. et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378, 595–605 (2011).
Servais, L. et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 32, 29–39 (2022).
Clemens, P. R. et al. Safety, tolerability, and efficacy of viltolarsen in boys with Duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 77, 982–991 (2020).
Shirley, M. Casimersen: first approval. Drugs 81, 875–879 (2021).
Bortolin, R. H. et al. Antisense oligonucleotide therapy for calmodulinopathy. Circulation 150, 1199–1210 (2024).
Mandegar, M. A. et al. CRISPR interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell 18, 541–553 (2016).
Limpitikul, W. B. et al. A precision medicine approach to the rescue of function on malignant calmodulinopathic long-QT syndrome. Circ. Res. 120, 39–48 (2017).
Lee, Y. et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 (2004).
Cai, X., Hagedorn, C. H. & Cullen, B. R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10, 1957–1966 (2004).
Singh, S., Narang, A. S. & Mahato, R. I. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm. Res. 28, 2996–3015 (2011).
Jackson, A. L. & Linsley, P. S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug. Discov. 9, 57–67 (2010).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug. Discov. 8, 129–138 (2009).
Dong, Y., Siegwart, D. J. & Anderson, D. G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug. Deliv. Rev. 144, 133–147 (2019).
Setten, R. L., Rossi, J. J. & Han, S. P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug. Discov. 18, 421–446 (2019).
Zhu, Y., Zhu, L., Wang, X. & Jin, H. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 13, 644 (2022).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Padda, I. S., Mahtani, A. U., Patel, P. & Parmar, M. Small interfering RNA (siRNA) therapy. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK580472/ (2024).
Solomon, S. D. et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation 139, 431–443 (2019).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Minamisawa, M. et al. Association of patisiran, an RNA interference therapeutic, with regional left ventricular myocardial strain in hereditary transthyretin amyloidosis: the APOLLO Study. JAMA Cardiol. 4, 466–472 (2019).
Habtemariam, B. A. et al. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin. Pharmacol. Ther. 109, 372–382 (2021).
Fontana, M. et al. Vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy. N. Engl. J. Med. 392, 33–44 (2025).
Balwani, M. et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 382, 2289–2301 (2020).
Fitzgerald, K. et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383, 60–68 (2014).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
Ray, K. K. et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: prespecified secondary end points in ORION 1. Circulation 138, 1304–1316 (2018).
Ray, K. K. et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 4, 1067–1075 (2019).
Ray, K. K. et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 382, 1507–1519 (2020).
Raal, F. J. et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med. 382, 1520–1530 (2020).
Garrelfs, S. F. et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N. Engl. J. Med. 384, 1216–1226 (2021).
Syed, Y. Y. Nedosiran: first approval. Drugs 83, 1729–1733 (2023).
Zhai, Y. et al. Clinical features of Danon disease and insights gained from LAMP-2 deficiency models. Trends Cardiovasc. Med. 33, 81–89 (2023).
Manso, A. M. et al. Systemic AAV9.LAMP2B injection reverses metabolic and physiologic multiorgan dysfunction in a murine model of Danon disease. Sci. Transl. Med. 12, eaax1744 (2020).
Grisorio, L., Bongianino, R., Gianeselli, M. & Priori, S. G. Gene therapy for cardiac diseases: methods, challenges, and future directions. Cardiovasc. Res. 120, 1664–1682 (2024).
Santiago Castillo, D. et al. Overexpression of cardiac calsequestrin as a novel gene-therapy approach to treat CPVT1: in silico and in vivo proves of principle. Eur. Heart J. 44, ehad655.3135 (2023).
Anttila, V. et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol. Ther. 31, 866–874 (2023).
Miake, J., Marban, E. & Nuss, H. B. Biological pacemaker created by gene transfer. Nature 419, 132–133 (2002).
Aistrup, G. L. et al. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc. Res. 83, 481–492 (2009).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Kingwell, K. Base editors hit the clinic. Nat. Rev. Drug. Discov. 21, 545–547 (2022).
Porto, E. M., Komor, A. C., Slaymaker, I. M. & Yeo, G. W. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug. Discov. 19, 839–859 (2020).
Naddaf, M. First trial of ‘base editing’ in humans lowers cholesterol — but raises safety concerns. Nature 623, 671–672 (2023).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Davis, J. R. et al. Efficient prime editing in mouse brain, liver and heart with dual AAVs. Nat. Biotechnol. 42, 253–264 (2024).
Wallace, E. et al. Long QT syndrome: genetics and future perspective. Pediatr. Cardiol. 40, 1419–1430 (2019).
Johansen, A. K. et al. Postnatal cardiac gene editing using CRISPR/Cas9 with AAV9-mediated delivery of short guide rnas results in mosaic gene disruption. Circ. Res. 121, 1168–1181 (2017).
Navarrete-Welton, A. et al. Low-efficiency gene editing is capable of suppressing arrhythmogenesis in long QT syndrome type II: computer simulation study. Circulation 148, A12241 (2023).
Liu, M. B., Priori, S. G., Qu, Z. & Weiss, J. N. Stabilizer cell gene therapy: a less-is-more strategy to prevent cardiac arrhythmias. Circ. Arrhythm. Electrophysiol. 13, e008420 (2020).
Bains, S. et al. KCNQ1 suppression-replacement gene therapy in transgenic rabbits with type 1 long QT syndrome. Eur. Heart J. 45, 3751–3763 (2024).
Dai, W. J. et al. CRISPR–Cas9 for in vivo gene therapy: promise and hurdles. Mol. Ther. Nucleic Acids 5, e349 (2016).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Chew, W. L. Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1408 (2018).
Dotzler, S. M. et al. Suppression-replacement KCNQ1 gene therapy for type 1 long QT syndrome. Circulation 143, 1411–1425 (2021).
Bains, S. et al. Suppression and replacement gene therapy for KCNH2-mediated arrhythmias. Circ. Genom. Precis. Med. 15, e003719 (2022).
Cheng, K. et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun. 5, 4880 (2014).
Qiu, J., Cai, G., Liu, X. & Ma, D. alphavbeta3 integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed. Pharmacother. 96, 1418–1426 (2017).
Spivak, M. Y. et al. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiology. EPMA J. 4, 20 (2013).
Zhang, J., Ma, A. & Shang, L. Conjugating existing clinical drugs with gold nanoparticles for better treatment of heart diseases. Front. Physiol. 9, 642 (2018).
Sahoo, S., Kariya, T. & Ishikawa, K. Targeted delivery of therapeutic agents to the heart. Nat. Rev. Cardiol. 18, 389–399 (2021).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Turnbull, I. C. et al. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol. Ther. 24, 66–75 (2016).
Castellani, C. et al. Poly(lipoic acid)-based nanoparticles as a new therapeutic tool for delivering active molecules. Nanomedicine 45, 102593 (2022).
Zhao, J. et al. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic. Res. Cardiol. 97, 348–358 (2002).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Fleury, S. et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation 107, 2375–2382 (2003).
Bongianino, R. & Priori, S. G. Gene therapy to treat cardiac arrhythmias. Nat. Rev. Cardiol. 12, 531–546 (2015).
Hacein-Bey-Abina, S. et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348, 255–256 (2003).
Penaud-Budloo, M. et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 82, 7875–7885 (2008).
Nathwani, A. C. et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 371, 1994–2004 (2014).
Nathwani, A. C. Gene therapy for hemophilia. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 569–578 (2022).
Buchlis, G. et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood 119, 3038–3041 (2012).
Wilson, J. M. & Flotte, T. R. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum. Gene Ther. 31, 695–696 (2020).
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298 (2018).
Flotte, T. R. E.-I.-C. Revisiting the ‘new’ inflammatory toxicities of adeno-associated virus vectors. Hum. Gene Ther. 31, 398–399 (2020).
Mingozzi, F. & High, K. A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 4, 511–534 (2017).
Tornabene, P. et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci. Transl. Med. 11, eaav4523 (2019).
Asokan, A. et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28, 79–82 (2010).
Weinmann, J. et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 11, 5432 (2020).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938.e22 (2021).
Louis Jeune, V., Joergensen, J. A., Hajjar, R. J. & Weber, T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods 24, 59–67 (2013).
Li, X. et al. Extracellular vesicle-encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation 148, 405–425 (2023).
Leborgne, C. et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 26, 1096–1101 (2020).
Elmore, Z. C., Oh, D. K., Simon, K. E., Fanous, M. M. & Asokan, A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight 5, e139881 (2020).
Schulz, M. et al. Binding and neutralizing anti-AAV antibodies: detection and implications for rAAV-mediated gene therapy. Mol. Ther. 31, 616–630 (2023).
Blaese, R. M. et al. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 270, 475–480 (1995).
Sibbald, B. Death but one unintended consequence of gene-therapy trial. CMAJ 164, 1612 (2001).
Lek, A. et al. Death after high-dose rAAV9 gene therapy in a patient with Duchenne’s muscular dystrophy. N. Engl. J. Med. 389, 1203–1210 (2023).
Horn, S. & Fehse, B. How safe is gene therapy? Second death after Duchenne therapy. Inn. Med. 65, 617–623 (2024).
Pacak, C. A., Sakai, Y., Thattaliyath, B. D., Mah, C. S. & Byrne, B. J. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet. Vaccines Ther. 6, 13 (2008).
Domenger, C. & Grimm, D. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum. Mol. Genet. 28, R3–R14 (2019).
Skopenkova, V. V., Egorova, T. V. & Bardina, M. V. Muscle-specific promoters for gene therapy. Acta Nat. 13, 47–58 (2021).
Jeon, J. Y., Ayyar, V. S. & Mitra, A. Pharmacokinetic and pharmacodynamic modeling of siRNA therapeutics — a minireview. Pharm. Res. 39, 1749–1759 (2022).
Lugin, M. L., Lee, R. T. & Kwon, Y. J. Synthetically engineered adeno-associated virus for efficient, safe, and versatile gene therapy applications. ACS Nano 14, 14262–14283 (2020).
Xie, Y., Sato, D., Garfinkel, A., Qu, Z. & Weiss, J. N. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys. J. 99, 1408–1415 (2010).
US National Library of Medicine. Phase IA and IB study of AAVrh.10hFXN gene therapy for the cardiomyopathy of Friedreich’s ataxia. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05302271 (2024).
US National Library of Medicine. A gene therapy study of RP-A501 in male patients with Danon disease. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06092034 (2025).
US National Library of Medicine. Multi-center, open-label, single-ascending dose study of safety and tolerability of TN-201 in adults with symptomatic MYBPC3 mutation-associated HCM (MyPEAK-1). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05836259 (2024).
US National Library of Medicine. A phase 1, dose escalation trial of RP-A601 in subjects with PKP2 variant-mediated arrhythmogenic cardiomyopathy (PKP2-ACM). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05885412 (2024).
US National Library of Medicine. Open-label, dose escalation study of safety and preliminary efficacy of TN-401 in adults with PKP2 mutation-associated ARVC (RIDGE-1). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06228924 (2025).
Ravindran, D. et al. Gene and cell therapy for cardiac arrhythmias. Clin. Ther. 42, 1911–1922 (2020).
Farraha, M., Kumar, S., Chong, J., Cho, H. C. & Kizana, E. Gene therapy approaches to biological pacemakers. J. Cardiovasc. Dev. Dis. 5, 50 (2018).
Kapoor, N., Liang, W., Marban, E. & Cho, H. C. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 31, 54–62 (2013).
Hu, Y. F., Dawkins, J. F., Cho, H. C., Marban, E. & Cingolani, E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 6, 245ra294 (2014).
Miake, J., Marban, E. & Nuss, H. B. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J. Clin. Invest. 111, 1529–1536 (2003).
Qu, J. et al. Expression and function of a biological pacemaker in canine heart. Circulation 107, 1106–1109 (2003).
Bucchi, A. et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation 114, 992–999 (2006).
Boink, G. J. et al. HCN2/SkM1 gene transfer into canine left bundle branch induces stable, autonomically responsive biological pacing at physiological heart rates. J. Am. Coll. Cardiol. 61, 1192–1201 (2013).
Protze, S. I. et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 35, 56–68 (2017).
Zhuang, R. Z., Lock, R., Liu, B. & Vunjak-Novakovic, G. Opportunities and challenges in cardiac tissue engineering from an analysis of two decades of advances. Nat. Biomed. Eng. 6, 327–338 (2022).
Bruegmann, T. et al. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 7, 897–900 (2010).
Arrenberg, A. B., Stainier, D. Y., Baier, H. & Huisken, J. Optogenetic control of cardiac function. Science 330, 971–974 (2010).
Jiang, C. et al. Cardiac optogenetics: a novel approach to cardiovascular disease therapy. Europace 20, 1741–1749 (2018).
Khawajakhail, R. et al. Advancements in gene therapy approaches for atrial fibrillation: targeted delivery, mechanistic insights and future prospects. Curr. Probl. Cardiol. 49, 102431 (2024).
Amit, G. et al. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation 121, 2263–2270 (2010).
Soucek, R. et al. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 9, 265–272 (2012).
Gaborit, N. et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 112, 471–481 (2005).
Fedida, D. et al. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ. Res. 93, 744–751 (2003).
Bikou, O. et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc. Res. 92, 218–225 (2011).
Igarashi, T. et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 125, 216–225 (2012).
Kunamalla, A. et al. Constitutive expression of a dominant-negative TGF-beta type II receptor in the posterior left atrium leads to beneficial remodeling of atrial fibrillation substrate. Circ. Res. 119, 69–82 (2016).
Trappe, K. et al. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur. Heart J. 34, 147–157 (2013).
Lau, D. H. et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation 119, 19–27 (2009).
Caporali, A. et al. Non-coding RNAs as therapeutic targets and biomarkers in ischaemic heart disease. Nat. Rev. Cardiol. 21, 556–573 (2024).
Lu, D. & Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 16, 661–674 (2019).
Wu, X., Reboll, M. R., Korf-Klingebiel, M. & Wollert, K. C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 117, 1257–1273 (2021).
Cannata, A., Ali, H., Sinagra, G. & Giacca, M. Gene therapy for the heart lessons learned and future perspectives. Circ. Res. 126, 1394–1414 (2020).
Rohatgi, R. K. et al. Contemporary outcomes in patients with long QT syndrome. J. Am. Coll. Cardiol. 70, 453–462 (2017).
Martinez, K. et al. Spectrum and prevalence of side effects and complications with guideline-directed therapies for congenital long QT syndrome. Heart Rhythm. 19, 1666–1672 (2022).
Denegri, M. et al. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ. Res. 110, 663–668 (2012).
Denegri, M. et al. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation 129, 2673–2681 (2014).
Bongianino, R. et al. Allele-specific silencing of mutant mRNA rescues ultrastructural and arrhythmic phenotype in mice carriers of the R4496C mutation in the ryanodine receptor gene (RYR2). Circ. Res. 121, 525–536 (2017).
Pan, X. et al. In vivo Ryr2 editing corrects catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 123, 953–963 (2018).
Bezzerides, V. J. et al. Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca2+/calmodulin-dependent kinase II. Circulation 140, 405–419 (2019).
Liu, B. et al. Gene transfer of engineered calmodulin alleviates ventricular arrhythmias in a calsequestrin-associated mouse model of catecholaminergic polymorphic ventricular tachycardia. J. Am. Heart Assoc. 7, e008155 (2018).
Matsa, E. et al. Allele-specific RNA interference rescues the long-QT syndrome phenotype in human-induced pluripotency stem cell cardiomyocytes. Eur. Heart J. 35, 1078–1087 (2014).
Lu, X. et al. RNA interference targeting E637K mutation rescues hERG channel currents and restores its kinetic properties. Heart Rhythm. 10, 128–136 (2013).
Cocera-Ortega, L. et al. shRNAs targeting a common KCNQ1 variant could alleviate long-QT1 disease severity by inhibiting a mutant allele. Int. J. Mol. Sci. 23, 4053 (2022).
Brunner, M. et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J. Clin. Invest. 118, 2246–2259 (2008).
Hamrick, S. K. et al. Single construct suppression and replacement gene therapy for the treatment of all CALM1-, CALM2-, and CALM3-mediated arrhythmia disorders. Circ. Arrhythm. Electrophysiol. 17, e012036 (2024).
Bains, S., Giudicessi, J. R., Odening, K. E. & Ackerman, M. J. State of gene therapy for monogenic cardiovascular diseases. Mayo Clin. Proc. 99, 610–629 (2024).
Yu, G. et al. Gene therapy targeting protein trafficking regulator MOG1 in mouse models of Brugada syndrome, arrhythmias, and mild cardiomyopathy. Sci. Transl. Med. 14, eabf3136 (2022).
Qi, M. et al. In vivo base editing of Scn5a rescues type 3 long QT syndrome in mice. Circulation 149, 317–329 (2024).
Kyriakopoulou, E. et al. Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy. Nat. Cardiovasc. Res. 2, 1262–1276 (2023).
Bradford, W. H. et al. Plakophilin 2 gene therapy prevents and rescues arrhythmogenic right ventricular cardiomyopathy in a mouse model harboring patient genetics. Nat. Cardiovasc. Res. 2, 1246–1261 (2023).
US National Library of Medicine. Gene therapy for ACM due to a PKP2 pathogenic variant. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06109181 (2025).
US National Library of Medicine. Study of AOC 1001 in adult myotonic dystrophy type 1 (DM1) patients (MARINA). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05027269 (2024).
Li, J. H., Liu, L. & Zhao, X. H. Precision targeting in oncology: the future of conjugated drugs. Biomed. Pharmacother. 177, 117106 (2024).
Patel, A., Zhao, J., Duan, D. & Lai, Y. Design of AAV vectors for delivery of large or multiple transgenes. Methods Mol. Biol. 1950, 19–33 (2019).
Mendell, J. R. et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 77, 1122–1131 (2020).
Blackwell, D. J., Schmeckpeper, J. & Knollmann, B. C. Animal models to study cardiac arrhythmias. Circ. Res. 130, 1926–1964 (2022).
Clauss, S. et al. Animal models of arrhythmia: classic electrophysiology to genetically modified large animals. Nat. Rev. Cardiol. 16, 457–475 (2019).
Odening, K. E. et al. ESC working group on cardiac cellular electrophysiology position paper: relevance, opportunities, and limitations of experimental models for cardiac electrophysiology research. Europace 23, 1795–1814 (2021).
Vermeulen, J. T. et al. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc. Res. 28, 1547–1554 (1994).
Nerbonne, J. M. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 525, 285–298 (2000).
Acknowledgements
S.B. thanks the Mayo Clinic Medical Scientist Training Program for fostering an outstanding environment for physician–scientist training. M.J.A. is supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program, Rochester, MN, USA.
Author information
Authors and Affiliations
Contributions
S.B. researched data for the article and wrote the manuscript. All the authors discussed its content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.B. and the Mayo Clinic have a license agreement with Solid Biosciences. M.J.A. is a consultant for Abbott, Boston Scientific, Bristol Myers Squibb, Daichii Sankyo, Illumina, Invitae, Medtronic, Tenaya Therapeutics and UpToDate. M.J.A. and the Mayo Clinic have license agreements with AliveCor, Anumana, ARMGO Pharma, Prolaio, Solid Biosciences and Thryv Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Lia Crotti, who coreviewed with Luca Sala, and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bains, S., Giudicessi, J.R., Odening, K.E. et al. Gene therapy for cardiac arrhythmias. Nat Rev Cardiol 23, 23–38 (2026). https://doi.org/10.1038/s41569-025-01168-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-025-01168-5